-

2021-12-03
how past pandemics relate to the current COVID-19 pandemic
-

2020-03-12
Choosing a university to go to is a life-changing choice. For most it is also a difficult one. With coronavirus restrictions in place, many students could not tour the schools they wanted to in person. It was because of this many schools began offering virtual tours. St. Mary's was one of these schools. I personally toured St. Mary's virtually. I didn't mind this option because I was already pretty sure I wanted to go there. The virtual tour was eye catching and easily accessible. I could see though how a virtual tour could be impersonal for others who were struggling to make a choice. It's not easy to capture an experience through a computer screen.
-
2021-12-03
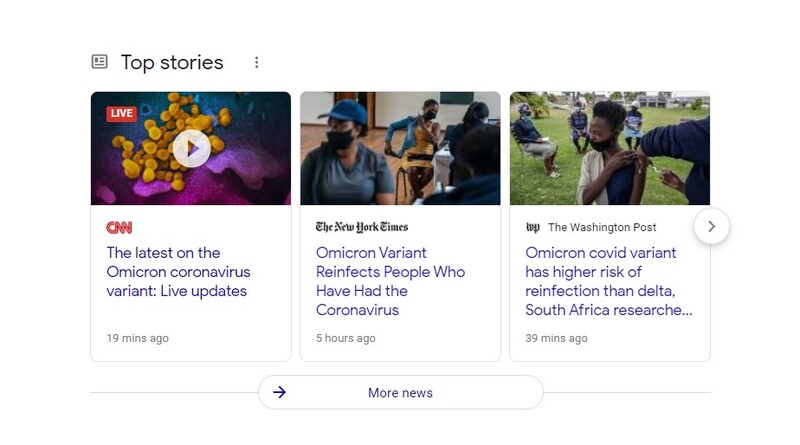
The new variant known as Omicron has started to appear in the U.S. as the WHO (World Health Organization) is more worried about its high number of mutations and labeled it as the "variant of concern."
-
2020-01-23
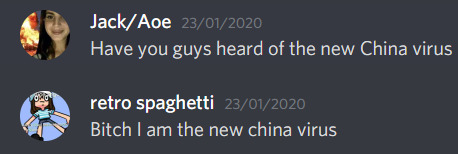
During January, my friends and I heard about the new virus and were fascinated. We joked and memed and speculated about it, however we never expected it to blow up this fast.
-

2021-11-29
This is an oral interview with two employees of the Office of Students retention at St. Mary's University. This showcases how the office of student retention played its part in guiding the transition from in-person to online and back to in-person classes throughout the pandemic. Their dedication to supporting students throughout the pandemic enriched the student experience for rattlers coming back to campus after an all online semester.
-

2020-08-11
I think I speak for most students when I say that when we were sent home during Spring 2020, I was confused about how we were supposed to go about things from now on. Personally, that confusion increased when they announced we would be returning virtually for Fall 2020. What about the students with younger siblings? What about the students who had sick family members? What about the students who had to get another job when their parents lost theirs? What about the students who were not able to access the internet? The attendance policy created specifically for Fall 2020 displays the different accommodations the university was able to provide for students asking these questions as well as many others that pertained to their specific situation. It was a tough time for everyone and each of us had our unique circumstances. This is one of the ways our university showed their support in the best and only way they knew how.
-

2021-11-30
The sometimes overlooked part of this pandemic has been the experience of the educators and their side of the story. Here I sat with my mom to gather her experience and thoughts on what's been a rough year. We talked about the changes she witnessed and how it affected not just the kids but herself and the teachers around her as well.
-

12/09/2020
C19OH
-

05/21/2020
University of Wisconsin- Eau Claire student Jack Nord interviews a Minneapolis-based six-grade teacher, Sue Buettgen. In this interview, Sue discusses her initial feeling when she first heard about the COVID 19 pandemic and how it changed her day-to-day routines. She discusses her transition from classroom to online teaching and all the new struggles that presented. She talks about her fears for her student’s safety and their individual home environments are affecting them. Sue dives into discussing social disparities and how the pandemic has highlighted the issue. Sue also discusses science experiments that she was trying to still make fun for her students and how her community has come together to help others. The interviewer, Jack Nord, also chimes in to briefly discuss his life as a college student. They both discuss farming and agricultural problems that have arisen. Sue finishes off by discussing how her home life has been impacted, how her family is coping and keeping safe. She discusses her hopes for the future before ending the interview.
-

09/30/2020
This interview consists of a perspective of a white male from Vermont living in Florida for the school year, whom has experienced COVID from the rural suburbs of Vermont, to the maskless warzone that is Florida. His perspective is one from a gamer, only knowing the interviewer though playing videogames together.
-

12/11/2020
Professor Damir Kovacevic was born in Bosnia before coming to the United States. He has lived across the midwest but currently lives in Eau Claire, Wisconsin. Damir works as an assistant professor of political science at the University of Wisconsin-Eau Claire with a focus on international relations. In this interview, Damir Kovacevic discusses how the pandemic has affected his life, profession, and emotions. Damir provides insight into how the University of Wisconsin-Eau Claire has handled the pandemic with testing and closing the college for the remainder of the Fall 2020 semester. Damir also touches on how Eau Claire, the state of Wisconsin, the United States, and foreign countries on the international scale handled the pandemic. He discusses how teaching as a career has changed and adapted to the pandemic. He discusses topics such as the media and misinformation when it comes to healthcare and the virus, but also the general decay in trust.
-
12/11/2020
The contributor of this item did not include verbal or written consent. We attempted to contact contributor (or interviewee if possible) to get consent, but got no response or had incomplete contact information. We can not allow this interview to be listened to without consent but felt the metadata is important. The recording and transcript are retained by the archive and not public. Should you wish to listen to audio file reach out to the archive and we will attempt to get consent.
-

05/26/2020
In this oral history interview, Alexander Michalski interviews Mike Michalski in Pewaukee, Wisconsin. Mike discusses his job and how it was affected by covid, the impact the virus has had on his friends and family, and home life. He touches on media and how the news is covering the virus. He also discusses local and federal government responses to the virus as well as his hopes for the future.
-

12/11/2020
The contributor of this item did not include verbal or written consent. We attempted to contact contributor (or interviewee if possible) to get consent, but got no response or had incomplete contact information. We can not allow this interview to be listened to without consent but felt the metadata is important. The recording and transcript are retained by the archive and not public. Should you wish to listen to audio file reach out to the archive and we will attempt to get consent.
-

05/22/2020
In this interview by Karen Kilby, a government contracts manager Nancy Campbell discusses how the COVID 19 virus has affected her life. She discusses the toilet paper shortage, the changes in her family dynamic, social isolation and the shortage of hand sanitizer and cleaners. Nancy also discusses her life as a senior citizen living in a rural area, the economy and her opinions on how government has delt with the virus.
-

2021-11-30
In order to get a better understanding of the situation in schools during these trying times I felt that we needed to talk to teachers. Getting their side of the story is just as vital as talking with students because in many cases they were just as new to this virtual world as us. So I sat down with my dad who has been a teacher for 20 plus years. I wanted to get his perspective on the situation and talk with him about how teachers felt during this period of transition.
-

2021-12-01
This image adds to my exhibit, how St. Mary's University wants their community to stay healthy and they support student and faculty needs throughout the pandemic.
-

2021-11-29
This is an audio interview of Zaragoza director Sanaa Abid. She gave a behind the scenes looks into how the student orientation was organized working with other campus facilities to ensure a safe program for students and parents. She offered insight as her roles from a new student, a student leader, and a student director.
From this interview, we are able to gain a better understanding of what the directors did during Zaragoza and how they accommodated to Covid-19. Students were able to still have an experience that allowed them to connect with other students and their families while still having a safe experience. From this interview, we can tell that the St. Mary’s Community came together to understand the different actions that were taken to ensure the program ran smoothly. Furthermore, Sanaa offered her unique experience from attending Zaragoza as a new student prior to Covid, a first time Zaragoza leader during 2020, and finally as Zaragoza Director during the summer of 2021.
-
2021-11-29
The microphone is an image I decided to add in for visuals that which accompanies the oral history that was conducted.
-

2021-11-17
The interview I conducted was with an individual who was prior military -- lived through the pandemic while enlisted and upon returning home what his experience was like with COVID 19 now that he has returned home-as a civilian.
Face to face interview. This interview allowed me gain more insight to learn how other people really feel about the pandemic and how they survived and are thriving throughout the lifespan of COVID.
-

2021-11-29
I interviewed a co-worker from S. Mary's University and wanted to get her input on how this pandemic has affected her from working from home and getting adjusted to coming back on campus. Angelica Coronado, has been employed with St. Mary's for about two years now. When she began working here the pandemic hit and she was not prepared for what was to come at all like so many others. Getting adjusted to not coming to work every day changed for everyone and getting costumed to working online was hard on some people. But after a year once again everyone was back at work in campus and trying to keep the campus safe people were required to get vaccinated. But most importantly trying to get back to normal and making sure the students felt safe is St. Mary's priority.
-

2021-11-29
This is an interview with Zaragoza Leader Jacqueline Mendez about her experience as a student orientation leader during the summer of 2021. She describes her role as a Zaragoza leader and how her student orientation experience prior to the pandemic differed from those of her students during the summer with COVID-19.
This interview offers personal insight to the effects of the pandemic as a new student attending St. Mary's. Jackie was able to connect her experience as a leader during the pandemic to her time enjoying Zaragoza as a new student prior to the pandemic. She describes the precautions that the university took during the summer during June of 2021 and August of 2021 when variants of the virus were changing and the state adopted different CDC guidelines.
-

2021-07-29
This screenshot of an Instagram post by the University that informs students to check for updates in their emails regarding safety procedures for Zaragoza days during the summer. The item was originally created by the StMU Rattler Family Instagram.
This Instagram posts shows the University's attempt to reach out to the St.Mary’s community advising them about safety precaution for the summer. Prior to the pandemic, events for Zaragzoa were not required to consider maks requirements, vaccination and health test records, or social distancing. However, with the priority of keeping the student staff and new students safe, the University used social media as a way to adequately reach out to the community to inform them of where they can find updates about safety precautions. They did this by posting a simple visual that showed a rattler wearing his mask and asking students to check on their email for updates.
-
2021-08-10
This screenshot of Zaragoza leaders training for their upcoming Zaragoza days during the summer of 2021. These students were wearing masks accommodating to COVID-19 guidelines. The item was originally posted by the Zaragoza Leaders Instagram, a page used to safely reach out to students.
This Instagram post shows Zaragoza team leaders following COVID-19 guidelines established by the university. In the days leading up to Zaragoza Orientation, team leaders were required to attend planning events to ensure the proper execution of safe orientation days for students and parents. Through this post, Zaragoza leaders were able to reach out to students and the St. Mary’s community in a safe and timely matter. Despite the restrictions brought to college campuses by the pandemic, St. Mary’s was able to find creative ways to remain connected to the community
-
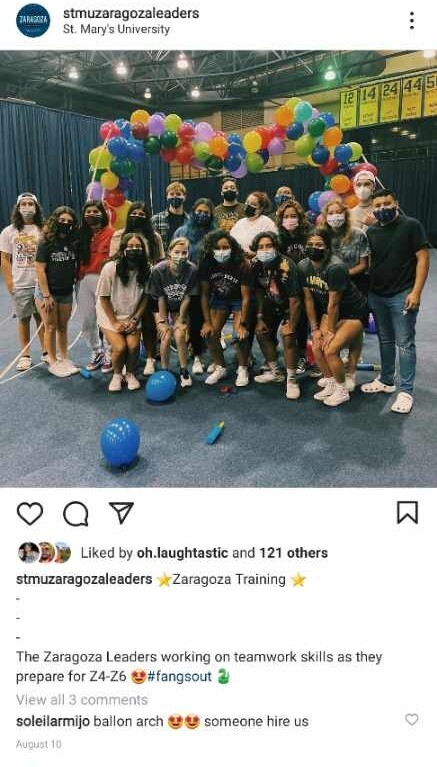
2021-08-10
This item is a screenshot of Zaragoza leaders training for their upcoming Zaragoza days during the summer of 2021. These students were wearing masks accommodating to COVID-19 guidelines. The item was originally posted by the Zaragoza Leaders Instagram, a page used to safely reach out to students.
This Instagram post shows Zaragoza team leaders following COVID-19 guidelines established by the university. In the days leading up to Zaragoza Orientation, team leaders were required to attend planning events to ensure the proper execution of safe orientation days for students and parents. Through this post, Zaragoza leaders were able to reach out to students and the St. Mary’s community in a safe and timely matter. Despite the restrictions brought to college campuses by the pandemic, St. Mary’s was able to find creative ways to remain connected to the community.
-

2021-11-09
Dr. Sara Ronis, a theology professor at St. Mary’s University, gives us a faculty perspective on how she believes the university handled the COVID-19 pandemic. She feels that despite being in such difficult positions, the university made the decisions they knew would be best for the St. Mary’s community. As a professor, she immediately thought of her students when COVID cases began to rise and the possibility of being sent home became an even more real possibility. She admires how St. Mary’s students, new and returning, have adapted to these new learning environments.
-

2021-11-29
This is one of many COVID 19 testing centers. We were fortunate to have this testing site made right here on St. Mary's University campus.
-

2021-11-29
The public was or maybe still is a lot more fearful of contracting COVID 19. If you were sick at you were advised to stay at home and work due to fear of contracting the virus. This image identifies some ways to know if you or someone may be infected with COVID 19.
-

2021-09-22
This is an excerpt from a video interview with Sister Grace that I and another student in my class did for a work project. Sister Grace is the Chaplin of the Law School at St. Mary’s University. Sister Grace graduated from St. Mary’s 1978 and worked in the undergrad Ministry and by 1993-94 in Law Ministry. Sister Grace has enjoyed watching students grow and succeed to becoming great people for the community. I used this excerpt because Sister Grace is someone who helps out the students and community and during the pandemic, she met a lot of people that ere going through hard times. Her and the church got together to help distribute food and clothing to the homeless, or whoever needed aid during the pandemic. She wanted to make sure everyone in the community was taken care of. Also, she even talks about how she got plenty of time to do more things during quarantine.
-

2021-11-29
I chose this image because it played a huge part of our reality in surviving the pandemic. By taking appropriate precautions and maintaining our distance with others in order to decrease the spread of germs and limit the spread of COVID 19. This image originated mainly from the CDC and every place a of business conformed to incorporate special mandates made by the government to help prevent the spread of COVID19 -- safely.
-
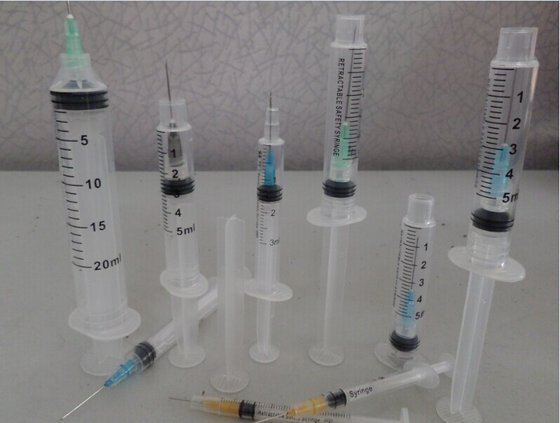
2021-11-29
Not everyone is a fan of hypodermic needles ( personally I am not). Healthcare has made considerable accommodations for those who are available for vaccinations by changing the size of the needle gauge; child and adult.
By the healthcare industry developing new, different, and special accommodations for providing vaccinations via needle sticks are taking into consideration not everyone likes needles. For the most the public have a fear of needles. To now make the vaccinations a lot more welcoming for you as child /adult have options. There are now even needle-less "sticks"
-

2021-11-11
Chelsea Osorio is a commuter student at St. Mary’s University and among the many personally affected by the COVID-19 pandemic. In her interview she highlights key points about what changes she noticed around the university that were used to protect our community. From a student perspective, she explains how safe she felt at school, especially with all the guidelines that were put in place. This goes to show how St. Mary’s students, faculty, and staff were committed to ensuring not only a healthy online environment but also making sure we returned to a safe and healthy community.
-

2021-11-22
It is obvious that the covid-19 pandemic has changed the college experience for all students. However, what was it like for those who do not know a pre-covid college experience. For some students, all they know is a covid campus. For Amanda Swan, a first-year resident assistant, her unique experience and the pandemic have allowed her to better relate to her residents. Having experienced a senior year of high school online and isolated gave makes allows her to better understand residents who have had similar experiences. Many residents who have not been on campus or have not been given the opportunity to experience a pre-covid college semester have been left to readjust to more social life. On top of many responsibilities of a resident assistant and academic duties, Amanda Swan is a very involved student navigating her way through college. Despite being her first time as a resident assistant and her first time living on campus, Amanda Swan has been able to serve as a resource for residents at St. Mary’s University.
-

2020-08-12
Following the reopening of schools through the virtual world a number of students across the country were faced with a new problem. They lacked the technology needed to attend their online class. Schools who fell under the title 1 classification , which is where children from low-income families make up at least 40 percent of the enrollment, were disproportionately affected by this problem. These families which often consisted of more than one child simply couldn't afford multiple computers. As a result many kids were still unable to attend their classes or do any work at all. This lack of technology was a problem that not only younger kids faced. Students ranging from all ages had to adapt and make due with whatever technology they had or were forced to go out to buy another computer.So in order to help fix the problem for younger students schools began to hand out chromebooks and ipads. By providing them with the technology to access their new classroom setting they could begin attending school again. While there were still other problems such as the lack of internet, handing out chromebooks and ipads definitely had a positive impact by providing a number of students with these new school supplies.
-

2021-08-16
After attending college online and having virtual classes, many students were eager to come back to the St. Mary’s University community. However, creating a community has looked different from pre-covid semesters. Before the pandemic hit, student life and university programming council hosted many events. Residence life and resident assistants also hosted events for residents in the dorm halls. When everything was virtual resident assistants tried engaging with residents and creating community online. This consisted of watching movies, playing games, or just having a space to talk to each other. Now that residence halls are open and most classes are in-person, creating community looks a bit more like pre-covid semesters. Resident assistants are required to create engagement opportunities for their residents. There are several ways to do this like traditional planned programs, spontaneous get-togethers, bring-along events, etc. Although the programs resemble pre-covid semesters more than the last three semesters, covid is still something to beware of. When planning events, resident assistants consider how many people might attend, whether the space is indoors or outdoors, will there be food, etc. Resident assistants are also required to uphold and enforce covid policies like mask-wearing. So, while also trying to create a sense of community, residence life and student staff still try to create a safe environment.
-
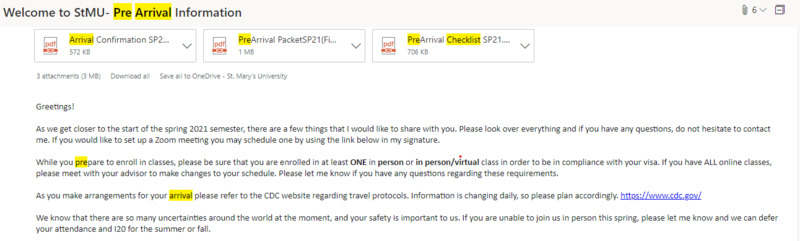
2020-01-05
During COVID-19 in 2020, some international students could not come to St. Mary’s due to closed embassies and lack of resources. In Spring 2021, international students came to continue their studies after studying online in their first semester. Days before coming to the U.S, international students received an email letting them know what they need to complete before arriving at the university. For some international students, this symbolizes the beginning of a new journey and the start of their experience at St. Mary’s University.
-

2021-11-23
Maria Del Mar Aponte Rodriguez is the new Director for the Center of International Programs at St. Mary’s University. After a semester of being in charge, Aponte tell us more about her experience as the new leader in the office and her future goals to make the CIP Office more open and warming for international students. As COVID has impacted several levels of the office, Aponte comments how she overcome these issues and how she is preparing a new plan of action for Fall 2022.
-

2021-11-22
Paula Ferradas Hiraoka is an international student at St. Mary’s University from Lima, Peru. After applying last year to St. Mary’s, Ferradas has come to the U.S to start her dream to become a good professional. In this interview we ask her experience as a new international student and how the resources in the university have given her the possibility to grow.
-

2021-11-11
As an international student, I believe international professors also had it rough during the pandemic. For that reason, I interviewed Dr. Cortina, a professor at St. Mary's University. To show students that we all have a story to share with the world.
-

04/20/2021
Alexis is a senior at St. Marys College majoring in political science. In this monologue she discusses her initial reaction to the COVID 19 pandemic, her employment status and how it was affected, and the economical effects she has witnessed due to the pandemic. She also discusses her family and home dynamic and her siblings response to online learning. She touches on social issues like racism and politics effect on society. She describes how she felt when she contracted COVID and how she spread it to her family. This monologue ends with her last thoughts and hopes for the future.
-

04/18/2021
C19OH
-

2020-10-06
C19OH
-

2020-10-06
C19OH
-

2020-10-06
C19OH
-

2020-09-19
C19OH
-

2020-08-26
C19OH
-

2020-07-11
C19OH
-

06/02/2020
The contributor of this item did not include verbal or written consent. We attempted to contact contributor (or interviewee if possible) to get consent, but got no response or had incomplete contact information. We can not allow this interview to be listened to without consent but felt the metadata is important. The recording and transcript are retained by the archive and not public. Should you wish to listen to audio file reach out to the archive and we will attempt to get consent.
-
2020-06-01
The contributor of this item did not include verbal or written consent. We attempted to contact contributor (or interviewee if possible) to get consent, but got no response or had incomplete contact information. We can not allow this interview to be listened to without consent but felt the metadata is important. The recording and transcript are retained by the archive and not public. Should you wish to listen to audio file reach out to the archive and we will attempt to get consent.
-

2020-05-29
C19OH
 2021-12-03
2021-12-03 2020-03-12
2020-03-12 2021-12-03
2021-12-03 2020-01-23
2020-01-23 2021-11-29
2021-11-29 2020-08-11
2020-08-11 2021-11-30
2021-11-30 12/09/2020
12/09/2020 05/21/2020
05/21/2020 09/30/2020
09/30/2020 12/11/2020
12/11/2020 12/11/2020
12/11/2020 05/26/2020
05/26/2020 12/11/2020
12/11/2020 05/22/2020
05/22/2020 2021-11-30
2021-11-30 2021-12-01
2021-12-01 2021-11-29
2021-11-29 2021-11-29
2021-11-29 2021-11-17
2021-11-17 2021-11-29
2021-11-29 2021-11-29
2021-11-29 2021-07-29
2021-07-29 2021-08-10
2021-08-10 2021-11-09
2021-11-09 2021-11-29
2021-11-29 2021-11-29
2021-11-29 2021-09-22
2021-09-22 2021-11-29
2021-11-29 2021-11-29
2021-11-29 2021-11-11
2021-11-11 2021-11-22
2021-11-22 2020-08-12
2020-08-12 2021-08-16
2021-08-16 2020-01-05
2020-01-05 2021-11-23
2021-11-23 2021-11-22
2021-11-22 2021-11-11
2021-11-11 04/20/2021
04/20/2021 04/18/2021
04/18/2021 2020-10-06
2020-10-06 2020-10-06
2020-10-06 2020-10-06
2020-10-06 2020-09-19
2020-09-19 2020-08-26
2020-08-26 2020-07-11
2020-07-11 06/02/2020
06/02/2020 2020-06-01
2020-06-01 2020-05-29
2020-05-29