Items
Tag is exactly
Moderna
-
 2022-06-17
2022-06-17Shots for the Youth
This article details the FDA's approval for vaccinations of children and infants. With encouragement from the Biden Administration, the youth are the final group with full approval for vaccinations against COVID-19. While this is a good thing for many who take the pandemic seriously, many Arkansans and southerners in general will likely be resistant to this new approval. The south has largely been skeptical of the vaccine and of COVID, and indeed the vaccinating of children with be a harsh topic across the south. It will be interesting to see the backlash and discussion from this decision, as well as analyzing the many questions that will doubtlessly be raised about the powers of the parents. -
 2022-06-17
2022-06-17The FDA authorizes COVID-19 shots for infants and preschoolers
This is a news story from NPR by The Associated Press. The United States has authorized the use of vaccines for infants and preschoolers. The Center for Disease Control and Prevention (CDC) is debating how the vaccines are to be administered. The article says that studies support giving these age groups the vaccines, as they are said to be effective and have minor side-effects. -
 2022-05-05
2022-05-05FDA restricts J&J’s Covid vaccine due to blood clot risk
This is a news story from NBC News by The Associated Press. Due to new findings, the J&J vaccine has been restricted by the FDA due to blood clot risks. It is not to be given to anyone unless they can't receive a different vaccine. Americans are now recommended to only be using Pfizer or Moderna shots instead. -
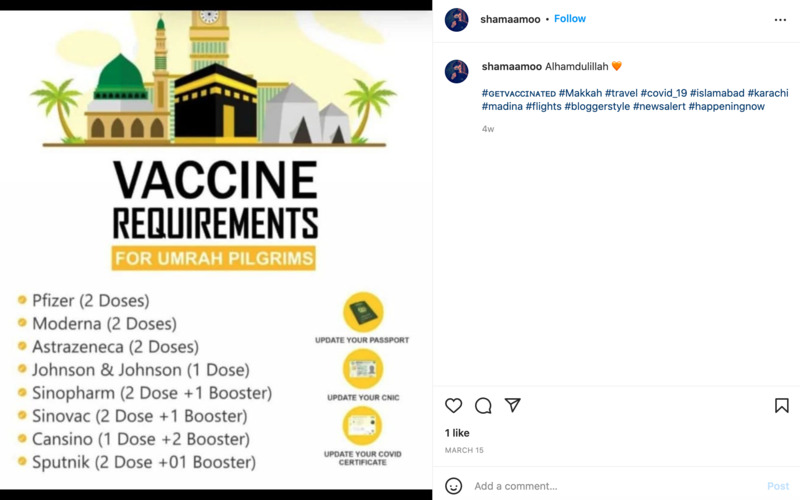 2022-03-15
2022-03-15Vaccine Requirements for Umrah Pilgrims
This is an Instagram post by shamaamoo. This is a PSA for vaccine requirements for those going on a pilgrimage. It shows a variety of vaccines that are accepted for this, as it includes many people from around the world. Pilgrimages have been affected by the virus since they include lots of travel and spaces with lots of people in one area. -
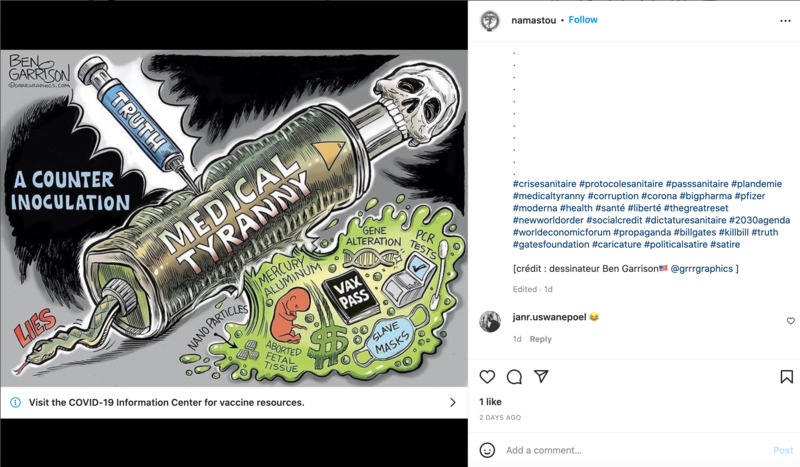 2022-04-05
2022-04-05Truth Serum
This is an Instagram post by namastou, and is partly in French. The comic posted in addition to the caption is by Ben Garrison, a political comic artist based in the United States. The comic shows how "truth" is being injected into "medical tyranny", and with that, the truth is that the masks are akin to making you slaves to the government, the vaccines cause gene alteration, and that PCR tests can come out as a false positive. -
 05/07/2021
05/07/2021Holly Tremble Oral History, 2021/05/07
Holly Tremble lives in Hudson, Wisconsin a suburb of the Twin Cities and is currently unemployed but also is a care worker once a week at a nursing home in Northfield, Minnesota so that she can see her father during this pandemic. In this interview, Holly discusses how COVID-19 has affected her life, her employment status, and family and community life. She shares what it has been like to go through this pandemic as well as the different approaches to the pandemic that she experienced being on the border of Minnesota and Wisconsin and the difference in policies in the area. -
 2020-07-14
2020-07-14Moderna Phase 1 results show coronavirus vaccine safe, induces immune response
Moderna Inc’s experimental vaccine for COVID-19 showed it was safe and provoked immune responses in all 45 healthy volunteers in an ongoing early-stage study, U.S. researchers reported on Tuesday. Volunteers who got two doses of the vaccine had high levels of virus-killing antibodies that exceeded the average levels seen in people who had recovered from COVID-19, the team reported in the New England Journal of Medicine. No study volunteers experienced a serious side effect, but more than half reported mild or moderate reactions such as fatigue, headache, chills, muscle aches or pain at the injection site. These were more likely to occur after the second dose and in people who got the highest dose. Experts say a vaccine is needed to put an end to the coronavirus pandemic that has sickened millions and caused nearly 575,000 deaths worldwide. Moderna was the first to start human testing of a vaccine for the novel coronavirus on March 16, 66 days after the genetic sequence of the virus was released. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, whose researchers developed Moderna’s vaccine candidate, called the results “good news,” noting that the study found no serious adverse events and the vaccine produced “reasonably high” levels of virus-killing or neutralizing antibodies. “If your vaccine can induce a response comparable with natural infection, that’s a winner,” Fauci said in a telephone interview. “That’s why we’re very pleased by the results.” Moderna shares jumped more than 15% in after-hours trading on Tuesday. The U.S. government is supporting Moderna’s vaccine with nearly half a billion dollars and has chosen it as one of the first to enter large-scale human trials. A successful vaccine could be a turning point for Cambridge, Massachusetts-based Moderna, which has never had a licensed product. Moderna’s shot, mRNA-1273, uses ribonucleic acid (RNA) - a chemical messenger that contains instructions for making proteins. When injected into people, the vaccine instructs cells to make proteins that mimic the outer surface of the coronavirus, which the body recognizes as a foreign invader, and mounts an immune response against. The results released Tuesday involved three doses of the vaccine, tested in groups of 15 volunteers aged 18-55 who got two shots, 28 days apart. The groups tested 25, 100 or 250 micrograms of the vaccine. Adverse events after the second dose occurred in seven of the 13 volunteers who got the 25-microgram dose, all 15 participants who received the 100 microgram dose and all 14 who got the 250 microgram dose. In the highest-dose group, three patients had severe reactions such as fever, chills, headache or nausea. One of these had a fever of 103.28 Fahrenheit (39.6 C). “We didn’t see any events that are characterized as serious adverse events,” said lead author Dr Lisa Jackson of Kaiser Permanente Washington Health Research Institute in Seattle, referring to reactions that require hospitalization or result in death. In June, Moderna said it selected the 100-microgram dose for its late-stage study to minimize adverse reactions. At that dose, Moderna said the company is on track to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, starting in 2021, from the company’s internal U.S. manufacturing site and strategic collaboration with Swiss drugmaker Lonza. “It’s a good first step,” said Dr William Schaffner, a vaccine expert at Vanderbilt University Medical Center who was not involved in the study. “There’s nothing here that would inhibit one from going ahead to the Phase 2/Phase 3 trials,” he said. In April, Moderna expanded the Phase 1 trial to include adults over 55, who are more at risk of serious disease, with the aim of enrolling 120 volunteers. Moderna said it will follow study volunteers for a year to look for side effects and check how long immunity lasts. Moderna started its phase 2 trial in May and expects to start a phase 3 trial on July 27. Phase 1 trials aim to ensure a treatment is safe and help determine an effective dose. Phase 2 trials test a treatment in a larger group and get an early read on effectiveness. Phase 3 trials are conducted in a large group of individuals to confirm efficacy and identify rare side effects. Moderna’s Phase 3 trial will be conducted in 30,000 volunteers. -
 2020-05-21
2020-05-21HHS, AstraZeneca Speed COVID-19 Vaccine Development; First Doses Due in October
The Trump administration announced early today that HHS and AstraZeneca will collaborate on a coronavirus disease vaccine called AZD1222. A statement released from HHS said the partnership will make “at least 300 million doses” of the vaccine available, “with the first doses delivered as early as October 2020.” The vaccine is one originally developed at the University of Oxford; the university and AstraZeneca announced a global development agreement for the vaccine on April 30. In its own statement early today, AstraZeneca said a phase 1/2 clinical trial of the vaccine began last month to assess its safety immunogenicity and efficacy in over 1000 healthy volunteers, who are 18 to 55 years of age. These volunteers are all in the United Kingdom. Late-stage trials would begin in several countries based on these results, the statement said. According to HHS, the agreement between AstraZeneca and the Biomedical Advanced Research and Development Authority (BARDA), an agency within HHS, would essentially kick start manufacturing of the doses while phase 3 clinical studies are under way this summer, involving 30,000 volunteers in the United States. BARDA will spend up to $1.2 billion for research, technology transfer, and scaled-up manufacturing, Emergency use authorization or licensure from FDA would be needed for the vaccine to reach the public, the statement said. As for the timeline, “Early milestones enable BARDA and AstraZeneca to determine how the program progresses forward.” “This contract with AstraZeneca is a major milestone in Operation Warp Speed’s work toward a safe, effective, widely available vaccine by 2021,” said HHS Secretary Alex Azar. “Getting a vaccine to the American public as soon as possible is one part of President Trump’s multi-faceted strategy for safely reopening our country and bringing life back to normal, which is essential to Americans’ physical and mental well-being in so many ways.” “The Trump Administration is making multiple major investments in developing and manufacturing promising vaccines long before they’re approved so that a successful vaccine will reach the American people without a day wasted,” Azar said. Besides the BARDA agreement, AstraZeneca said it has reached deals with the Coalition for Epidemic Preparedness Innovations (CEPI), the Vaccine Alliance and the World Health Organisation (WHO), to ensure the fair allocation and distribution of the vaccine around the world. AstraZeneca is also in discussions the Serum Institute of India and other potential partners to boost production and distribution. AstraZeneca also holds a major stake in Moderna Therapeutics, which announced earlier this week its experimental vaccine had produced antibodies in small group of healthy volunteers. -
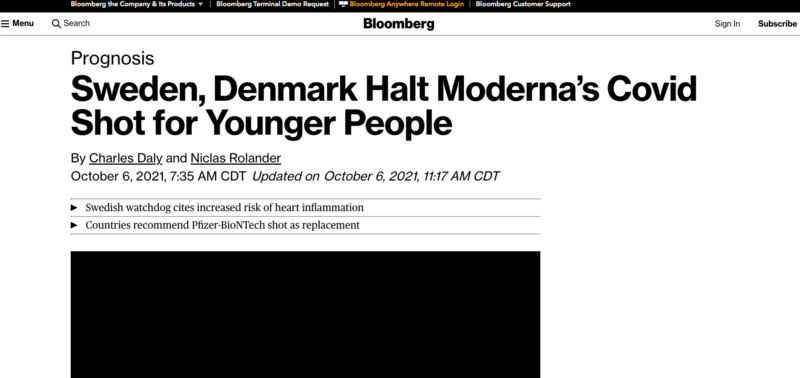 2021-10-06
2021-10-06Sweden, Denmark Halt Moderna’s Covid Shot for Younger People
Sweden and Denmark decided to halt vaccinations with Moderna Inc.’s Covid-19 shot for younger people because of potential side effects. -
 2021-05-14
2021-05-14Golden Gate Area Council Summer Camp 2021 Combined Leaders’ Guide (Version 2.1 - May 14, 2021)
This is a document from Golden Gate Area Council that provides a variety of information on the council's Scout camps, including Camp Wolfeboro. The guide is made for Scoutmasters and other adult leaders, hence the name of the document. Page 1 provides a summary of changes made to camps for the 2021 summer. Some of the changes the documents discusses for the 2021 year include: Page 10: Troops cannot arrive to camp early, on Saturday instead of Sunday. All troops must arrive on Sunday. Page 13: "ALL Campers must show proof of either a valid vaccination for COVID-19 (both doses of either the Moderna or Pfizer with at least two weeks of elapsed time after the second dose or two weeks of elapsed time after the one-dose Johnson & Johnson vaccine) or a negative COVID-19 test within 72 hours of arrival at camp." Page 14: Dining halls at camp would be at 50% capacity, with outdoor seating available. Page 14: Wednesday meals will not be given to troops to cook, but instead will be served in the Dining Hall like all other meals (specific to Camp Wolfeboro). Page 15: Visitors are not allowed at camp. Pages 20-21: Explains COVID-19 procedures before camp and at camp, including vaccination or testing requirements found on page 13. Page 24: All campfires are prohibited, except for propane- and butane-based firepits. Page 45: The Lifesaving merit badge will not be offered at Camp Wolfeboro in 2021. Page 49: The Adventures Connection Experience (ACE) program will not be offered (specific to Camp Wolfeboro). -
 2021-06-03
2021-06-03My Covid Vaccine Experience
These are the two Facebook posts I made the day I got my first shot and my second. I received the Moderna vaccine. As a teacher, I was able to get mine sooner than many others. Many people I know had (and still have) reservations about getting the vaccine. I believe in science. I believe in vaccines. It is disheartening and dangerous to see so many Americans throwing away an opportunity to protect themselves, their families, and their community because of politics. Science should not be political. The vaccine did make me feel ill, especially the second one, but it was temporary. I would do it a hundred more times if I had to. A friend of the family said they would not get the vaccine because, "What's in it for me? Even if I get Covid, I am young and healthy, unlikely to die." I found that statement alarmingly self-centered. Getting the vaccine isn't about you as individual as much as it is about you protecting your community and the world. As the saying goes nowadays, "Until all of us are safe, none of us is safe." -
 2021-04-21
2021-04-21Enjoying the Chaos Wastes while I wait for my second Covid shot
On the twenty first of April, a new free DLC for the video game Vermintide 2 released called Chaos Wastes. Like a previous video game I posted about in these archive which has occupied my time during the pandemic, Vermintide is set in the Warhammer Fantasy Universe. WHF is essentially Tolkien high fantasy turned up to eleven, more over the top in every way. Vermintide takes place during the End Times, a narrative event from the tabletop game from around 2014. The venerable franchise with 30+ years of writing and stories by that point was destroyed in real life by its replacement Warhammer Age of Sigmar, and in the story the world was finally consumed by the powers of Chaos. In Vermintide, teams of four players team up to fight the horrors that assail the Empire of Mankind right at the beginning of the End Times. This new DLC, focusing on an expedition straight into the heart of the Chaos Wastes, takes the game in a new narrative direction and ties it in more broadly with the End Times narrative itself. The Ubersreik 5, as the protagonist group is referred to after their exploits from the first game, is primarily opposed to two elements of disease and decay: the Skaven, human-sized rats that live in a massive Under-Empire that seek to spread plague and take over the surface, and the Norscans, basically fantasy power-metal viking marauders who worship the chaos god Nurgle, lord of decay and disease. Our protagonists travel to a fortress deep in the reality-warped wasteland near the North Pole in order to contact their respective gods to seek aid to combat the End Times. While they are not fighting fantasy characters straight out of the 1980s, modern scientists and healthcare professionals have been fighting a virus which has threatened us all in a global pandemic. I go to get my second shot of Moderna tomorrow, and while I have been enjoying this new DLC and embarking on heroic quests with my friends online, others have worked to allow people like me to finally protect ourselves from Covid with a vaccine. -
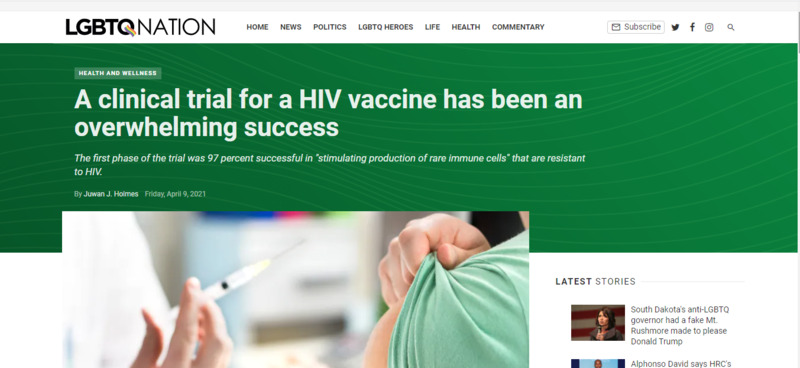 2021-04-09
2021-04-09Clinical Trials for HIV Vaccine has been Overwhelming Success due to the help of COVID-19 Vaccine
Clinical trials for HIV vaccines have been overwhelmingly successful with a 97% success rate at stimulating the production of rare immune cells which could lead to vaccines in the future. The COVID-19 vaccine has led to the increased development of m-RNA dosed vaccine which is also found in many other vaccines. By producing the COVID-19 vaccine has led to much more funding and research into the mRNA vaccine field which will bring about new changes in medicine in the future. -
 2021-04-16
2021-04-16Vaccine Booster Shots
This article is about the Moderna and Pfizer vaccine shots will likely require follow-up booster shots. The executives of both companies announced that it is likely that people that received the vaccine will need their first booster shot within 12 months of receiving the vaccine, then possibly yearly shots afterward. At the end of the article it does note that the Pfizer vaccine is still 93.1% effective 6 months after the vaccine and Moderna reports 90% effective after the 6 month period. However it still seems that we will have to receive booster shots, which is no big deal unless people have the side effects that they had from the original vaccine. This would absolutely prevent many people from following through or even receiving the original vaccine. -
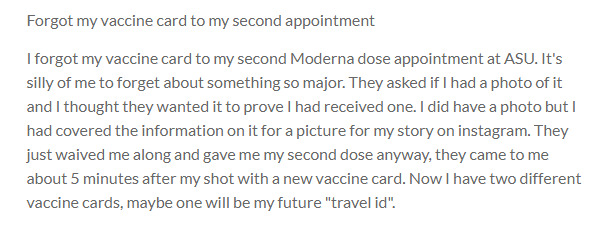 2020-04-13
2020-04-13Forgot my vaccine card to my second appointment
I forgot my vaccine card to my second Moderna dose appointment at ASU. It's silly of me to forget about something so major. They asked if I had a photo of it and I thought they wanted it to prove I had received one. I did have a photo but I had covered the information on it for a picture for my story on instagram. They just waived me along and gave me my second dose anyway, they came to me about 5 minutes after my shot with a new vaccine card. Now I have two different vaccine cards, maybe one will be my future "travel id". -
 2021-03-31
2021-03-31Shot One - Community Vaccination Clinic
I got my Moderna vaccine at the Branch- Hillsdale - St. Joseph Community Health Agency Vaccination Clinic held in Hillsdale Public High School. My first Moderna shot was on Wednesday, March 31, 2021. It was well run - I hardly had time to sit down. There was no waiting. It was held in a large gymnasium. Everyone was wearing masks, which is rare in this part of Michigan. I was relieved & quiet while I got my shot, just taking it in. Later that night my arm was sore at the injection site. I was also exhausted that evening, but I think the exhaustion was more of a psychological response to finally starting my vaccination journey, & not a side effect of the vaccine. My husband got his two weeks previously at a Rite Aid in Coldwater, Michigan. He also got a Moderna shot. -
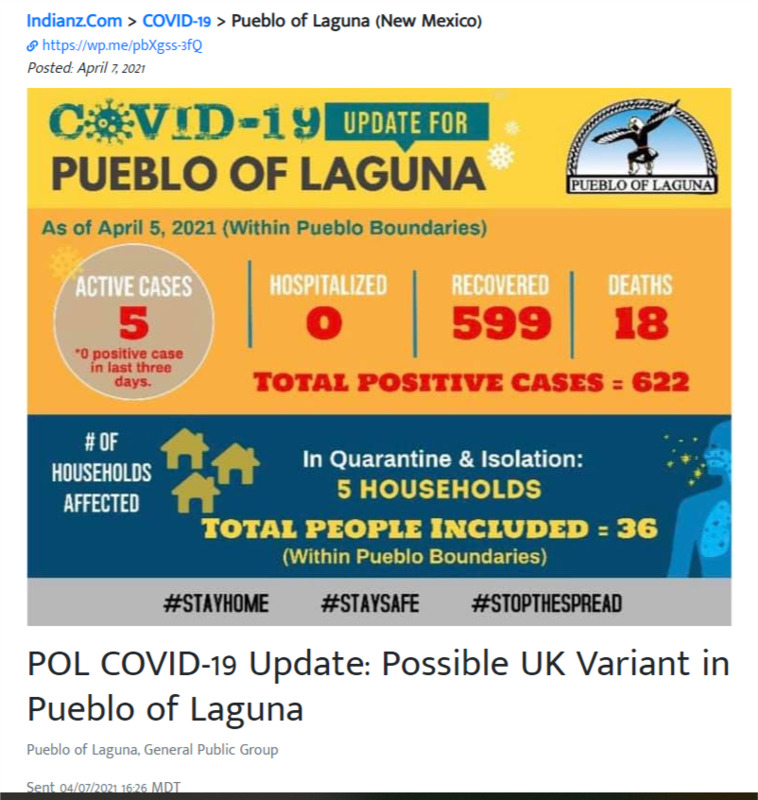 2021-04-07
2021-04-07POL COVID-19 Update: Possible UK Variant in Pueblo of Laguna
Both vaccine makers, Pfizer and Moderna, have tested the effectiveness of their vaccines against the UK variant and tests are showing the vaccines are effective against this strain. The POL EOC strongly encourages that we all take individual responsibility to get vaccinated to protect ourselves and our children who are still not able to receive the vaccine. The virus still has a hold of us and is putting our community at risk so we must continue to remain vigilant. -
 2021-04-01
2021-04-01News Article: ASU watching new COVID-19 'Arizona variant' with a mutation known to weaken vaccines
By Amanda Morris of the Arizona Republic: Arizona State University researchers have found a home-grown variant of the coronavirus emerging in Arizona that they say should be monitored closely because it carries a mutation known for weakening vaccines. In a non-peer reviewed study that published Sunday, researchers said they have detected 17 cases of the new variant since February, 15 of which were in Arizona. The other two cases were found in Houston in late February and New Mexico in early March, suggesting that the variant has begun to spread. "My hope is that we do not see more of these cases. The whole point of surveillance is to keep this from spreading," said Dr. Efrem Lim, an ASU virologist and assistant professor. The variant is known as the B.1.243.1 variant, and descends from a common lineage of the virus called B.1.243, which nationally makes up about 2.5% of all cases, according to David Engelthaler, director of the Translational Genomics Research Institute's infectious disease division in Flagstaff. "It's not dominant. But, there's a fair amount of that lineage that has been able to hang around," Engelthaler said. "It seems to have picked up this E484K mutation, what we call the 'eek!'" This E484K mutation has also been seen in the variants first detected in South Africa and Brazil, as well as one new variant recently discovered in New York. Numerous studies have shown that this mutation — located in the spike of the virus — lowers antibody responses to the virus and could weaken vaccines. Antibodies are one of the body's tool to recognize and fight the virus. The E484K mutation has been shown to weaken antibody responses. One study from Seattle showed that it caused the neutralizing effects of antibodies to decrease by tenfold, and numerous other studies have shown similar results. American vaccine development company Novavax reported that its COVID-19 vaccine was 96.4% effective against the original coronavirus strain and 86.3% effective against the U.K. variant, but was far less effective in South Africa, where the South Africa variant carrying this mutation is dominant. In the South Africa trials, the vaccine was shown to be 48.6% effective overall, and 55.4% effective in HIV-negative individuals. Moderna announced a sixfold reduction in antibody responses from its vaccine against the South Africa variant, and Pfizer observed a drop in vaccine-induced antibody responses against the South Africa variant. The Johnson & Johnson vaccine is reported to be 64% effective against moderate to severe COVID-19 in trials in South Africa vs. 72% effective in U.S. trials. Though the E484K mutation appears to reduce antibody response and possibly reduce vaccine efficacy, Lim stressed that vaccines still work well and said people should get their vaccines as planned. Scientists are monitoring mutations in the spike of the novel coronavirus. Community spread is a concern Though the new Arizona variant carries this mutation, it's still possible for the variant to fizzle out and stop spreading. Lim said researchers have found two other cases where viruses within the B.1.243 lineage independently picked up the E484K mutation, but did not spread. "In both cases, they never led to more transmissions," Lim said. Engelthaler has also tracked other lineages where the E484K mutation showed up, but those strains fizzled out. Overall, researchers have detected over 60 samples containing the E484K mutation statewide, according to TGen's Arizona COVID-19 sequencing dashboard. In order to continue spread, Engelthaler said variants need to be very "fit." "This mutation has popped up on multiple instances and then just goes away," he said. "This one mutation by itself doesn't give the virus superpowers." "It’s definitely a mutation of concern but time will tell if it will be a variant of concern," he added. If the mutation shows up in a more fit version of the virus, then Engelthaler said it becomes more of a concern. The new variant in Arizona is different than past cases because it has already spread from one person to another and could spread further, according to Lim. He said "one-off" mutations here and there are normal, but that the bigger question is about the transmission levels of this variant. A variant's ability to spread to others is also dependent on human behavior, Lim said. If people follow public health guidelines, they are less likely to spread variants to others. In total, Lim said the new Arizona variant has 11 mutations, which is "quite a bit more" than normal virus variations. These 11 mutations could be helping the virus survive or spread and could also act as a "fingerprint" to help researchers identify the new variant, Lim said. Another one of the 11 mutations is located in the spike that the virus uses to attach to and infect cells. Engelthaler said that because of the importance of the spike, any mutations in that area could affect things like how fast the virus spreads or how severe the related illness is. Both Lim and Engelthaler said it's too soon to tell whether the other mutations in this variant have any effect. Overall, this variant still seems to account for a very low percentage of overall cases in the state, according to Dr. Joshua LaBaer, the executive director of ASU's Biodesign Institute. ASU researchers wrote that it's still possible there are more undetected cases of the variant since there are limited efforts to genetically monitor the virus nationwide. In Arizona, roughly 1.3% of cases overall have been genetically sequenced, or analyzed, according to TGen's Arizona COVID-19 sequencing dashboard. In February and March, over 3% of cases were sequenced, higher than national rates of sequencing, which were below 1% in January. ASU is working with the Arizona Department of Health Services to monitor the new variant and hopefully prevent further spread through contact tracing and other public health measures, Lim said. California and UK variant cases rise Currently the Arizona variant is only considered a "variant of interest" and not a "variant of concern." These are different categories outlined by the CDC and used to assess the risk level of each variant. The CDC defines "variants of interest" as those that are associated with potential changes, whereas "variants of concern" have evidence showing actual changes such as increased transmission, more severe disease or antibody evasion. There are five variants of concern, which include variants first identified in the United Kingdom, South Africa, Brazil and California. Two variants from California were elevated from variant of interest to variant of concern this month and have rapidly spread in Arizona. "They're closely related to each other and have definitely been documented with increased transmissibility and some impact on some antibody treatment," Engelthaler said. In November 2020, both the California variants accounted for only 0.73% of Arizona's genetically sequenced samples. By March, they accounted for 31.64% of samples and are predominant variants statewide. One non-peer reviewed study from the University of California San Francisco showed weaker antibody responses against the California variants. Because of concerns that monoclonal antibody treatments may be less effective against these two variants, the U.S. Department of Health and Human Services announced two weeks ago that it would limit the distribution of one treatment to states with high levels of the California variant, including Arizona. The California Department of Public Health also recommended that the state stop distributing the treatment, which is made by American pharmaceutical company Eli & Lily. In a health alert, the department said this treatment was unlikely to be active against the California variants. The U.K. variant, which is highly contagious, has also been spreading statewide ever since it was first detected in late January. In March the U.K. variant accounted for 4.72% of genetically analyzed samples. Currently, Engelthaler said Arizona has over 100 cases of the U.K. variant and over 1,000 cases of the California variants. Arizona also detected its first cases of the South Africa variant last week. So far, Lim said that all of the variants of concern are manageable and have not risen to the level of "variant of high consequence," which the CDC defines as variants that are shown to significantly reduce the effectiveness of prevention and medical measures. "The risk is whether one of these current variants of concern acquire additional mutations that push it up to the next level," Lim said. To prevent further mutations, LaBaer said it's important to prevent spread of the virus by continuing to follow health guidelines and getting vaccinated. The more the community can prevent the spread of the virus, the less mutations will occur, he said. "We're kind of in this race right now between the developed dominance of these much more infectious variants that are now spreading throughout the country and getting people vaccinated," LaBaer said. "At the moment, I'm a little worried that the spread of this virus is so fast that that may outpace our ability to get vaccines in arms." He said it was theoretically possible that new variants could escape the vaccines, meaning that the public would move backward away from reaching herd immunity. But Lim said the vaccines can easily be updated to protect against new variants. Pharmaceutical companies like Pfizer and Moderna are already working on developing updated booster shots. In the meantime, researchers will continue to monitor the Arizona variant to see if it spreads further. Engelthaler said he expects the most fit variants of the virus to become more dominant statewide as people continue to get vaccinated and stamp out less successful strains. "There's a bit of a race here with the virus — a survival of the fittest race," Engelthaler said. "But what we don't want is to raise too much concern that things are going in the wrong direction...what we're doing is closely watching the evolution of a virus like we never have before. It's good that we have this capability, it's more important to put it into context." Amanda Morris covers all things bioscience, which includes health care, technology, new research and the environment. Send her tips, story ideas, or dog memes at amorris@gannett.com and follow her on Twitter @amandamomorris for the latest bioscience updates. Independent coverage of bioscience in Arizona is supported by a grant from the Flinn Foundation. -
 2021-03-04
2021-03-04What pandemic? One urbanite's weekend venture into rural Arizona
In addition to all the other aspects that currently define my life, I can almost see the end of my first year of graduate studies in Arizona State University's Global History program. I returned to academia in the fall of 2019, wrapped up 34 undergrad credit in 9 months with a 4.1 GPA, and started my master's studies in the fall of 2020. I still have to work a dayjob to keep the lights on, and I have a side hustle ghost writing fiction novels and hosting a podcast on creative writing. Time is my most valued and least possessed commodity. My school schedule is generally comprised of 7.5-week courses, and the university recommends taking no more than one at a time. I couldn't avoid doubling up during the first two months of this spring semester, and, to be candid, I arrogantly denied the validity of the university's guidance. By the end of the first term, I desperately needed to remember what a weekend felt like. Because God blessed me with the Greatest Wife in The History of the World, she scheduled a four-day weekend for us in the White Mountains in eastern Arizona. For those unfamiliar with the area, eastern Arizona has the largest stand of Ponderosa pine trees in the world. Hunters consistently harvest trophy elk and deer from the White Mountains and Gila National Forest, which spans the Arizona-New Mexico border. Unlike Colorado's coniferous forest, eastern Arizona seems devoid of pine beetle kill. Nothing but healthy, evergreen forest and the scent of sun-warmed pine greets you. We stayed in a vacation home on the outskirts of Pinetop, brought our groceries from home, and largely intended on hiking, cooking, drinking, and doing a lot of nothing. When we arrived in Pinetop in early March 2021, I had already fully recovered from COVID-19 and had time for both of my Moderna vaccines to have taken full effect. My wife had neither protective barrier, but we had generally become comfortable with purpose-driven shopping (as opposed to "window shopping") and takeout dining. As such, we stopped into a bakery to get breakfast on the way out to the hiking trails as a vacation treat. To our surprise, many of the patrons weren't wearing masks while walking through the restaurant or waiting in line. That made us a little uncomfortable. Then, one of the employees walked out from the kitchen with no mask on and began working on filling orders at the front, cold-food storage counters. Both of us panicked a bit and considered cancelling our orders and leaving. My wife pulled up the Arizona Department of Health Services site and quickly found that entire county had endured only a little more than 560 cases. A quick bit of division translated that into an average of two infections per day for the entire pandemic year-to-date. The statistical odds of the unmasked clerk or patrons presenting a health risk to either of us fell to just north of zero. NOT zero, but we both felt we could see it from there. The ham, egg, and cheese croissants were delicious, by the way. In trying to be good guests, we continued to wear our masks whenever we ventured into public spaces and businesses. Less than half of those around did the same, and I didn't see or hear anyone confront each other about mask wearing. Our last venture out that weekend was to a beer garden with a prominent outdoor patio and seating area. We again wore our masks inside the establishment, but we immediately felt like outcasts for having done so. When we stepped inside, it looked as though the town villain had just stepped through the saloon doors: all activity inside the business stopped, and everyone seated inside turned around to look us up-and-down for few silent moments. If anyone had been playing piano, they would have switched to a minor key. NO ONE else inside wore a mask, and the interior tables didn't appear to have been spaced to comply with prevailing social distancing guidelines. Everyone stayed kind of quiet until we ordered beers and asked to sit outside. In hindsight, I wonder if they expected we were there from some government bureaucracy to issue citations, or just out-of-towners about to have a value-based hissy fit? I have been generally opposed to broad behavior mandates that typically justify compliance on urban problems, but that weekend compelled me to really consider the divergent pandemic realities Arizonans have endured for the past year. Further analysis of county-specific data seems to suggest at least four divergent pandemic experiences within Arizona: urban centers, border counties, rural counties, and Native American reservations. I hope to better understand the personal experiences of those who lived in these diverse regions and how the pandemic affected their perspective and reality. -
 2021-03-25
2021-03-25News Article: Graham County (AZ) now with less than 150 active documented COVID-19 cases
"By Jon Johnson, jonjohnsonnews@gmail.com SAFFORD – Graham County has had very few new confirmed cases of COVID-19 in the past month, lowering its numbers to just 147 active cases as of Thursday. According to the Graham County Department of Health and Human Services, Graham County has had a total of 5,355 confirmed cases for the course of the pandemic, with 5,132 listed as being recovered, 147 active, and 76 deaths in more than a year. No new cases were recorded Thursday, and, according to the Arizona Department of Health Services COVID-19 school dashboard, Graham County had just a 1 percent positivity rate as of the week of March 14. That is good for a tie with Apache County for the second-lowest percent positivity rate out of Arizona’s 15 counties. Only Greenlee County, which registered a zero percent positivity rate from Feb. 27 – March 14, had lower. With the lower cases statewide and vaccine rollout, Governor Doug Ducey issued an Executive Order on Thursday, rolling back several COVID-19 mitigation measures involving businesses and gatherings. This comes as other states roll back their COVID-19 mitigation measures as well. The rollout of the various COVID-19 vaccines has picked up steam in the last month, with the state opening up the vaccine to anyone 16 years old or older for the Pfizer vaccine. Anyone 18 years old or older can be administered the Moderna and Johnson & Johnson vaccines. The San Carlos Apache Healthcare Corporation is holding a free, drive-through vaccine clinic on Saturday, March 27 at the San Carlos High School. No appointment is necessary. The clinic will be administering both the Pfizer and Moderna vaccines. Graham County and Greenlee County are also providing vaccination sites for those 18 and older, and provide the Moderna and Johnson & Johnson vaccines. Greenlee County: According to the Greenlee County Health Department, the county currently has just nine active cases of COVID-19. For the course of the pandemic, Greenlee County has had 568 confirmed positive cases (by far the lowest out of any of Arizona’s 15 counties), with 549 recovered cases, nine active, and 10 deaths." -
 2021-03-19
2021-03-19Post-vaccine handouts
After receiving the first dose of the Moderna vaccine, the volunteers at ASU handed out these booklets with information about the Moderna vaccines. It lets you know that no vaccine is FDA approved and that you should report any symptoms to the CDC using the vaccine safe program with your smartphone. -
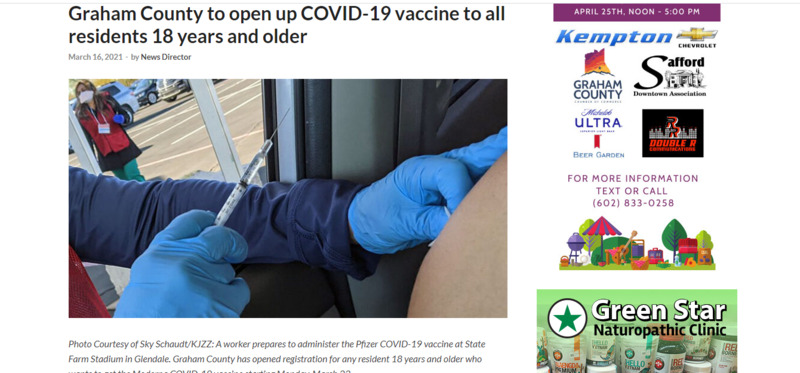 2021-03-16
2021-03-16Graham County (AZ) to open up COVID-19 vaccine to all residents 18 years and older
Staff Reports SAFFORD – The Graham County Department of Health and Human Services has announced that starting Monday, March 22, any resident of Graham County who is 18 years old or older will be eligible to receive a vaccine for COVID-19. Those who would like a COVID-19 vaccination should contact their primary care physician or schedule an appointment with the health department by clicking here. Those who schedule appointments will currently be given the two-shot Moderna vaccine at the Graham County Health Department Vaccination Center at 627 W. Main Street in Downtown Safford. The health department will announce a clinic for the one-shot Johnson and Johnson vaccine at a later date. While the Pfizer and Moderna two-shot vaccines utilize messenger RNA, the Johnson & Johnson vaccine works through a different mechanism and uses the more traditional DNA, which is introduced to the nucleus of cells with an adenovirus which is modified so it cannot replicate itself and cause disease. All three vaccines have been approved for use by the federal government and have safety records in good standing. All prompt the body to produce T-cells, which retain a memory of the protein and attack it. “We would like to thank everyone for their support as we have navigated through the COVID-19 pandemic this past year,” said Graham County Health Department Director Brian Douglas. Greenlee County Gila Health Resources in Greenlee County will hold a COVID-19 vaccine drive at the Morenci Club Hall at 314 Plaza Dr. in Morenci on Friday, March 19, from 1 – 8 p.m. At the vaccine drive, any adult resident of Greenlee County or those who work in Greenlee County can show up to receive a dose of the Moderna vaccine with no appointment or registration necessary. -
 2021-03-17
2021-03-17Phoenix among U.S. Sites for Moderna’s COVID-19 trials on children
By Jacob Holter/Cronkite News WASHINGTON D.C. – Children from 6 months up to 12 years old could soon start getting the COVID-19 vaccine in Phoenix as part of a trial of the drug’s effectiveness on young people. Drug-maker Moderna announced this week that Phoenix will be one of the cities where it will test smaller doses of its COVID-19 vaccine, which has currently only been approved for adult use, on preteens. The company has already started trials of the vaccine on teenagers. While children have proven to be less susceptible to the disease, health experts say it’s important to have the option of a vaccine for younger kids as schools reopen and to improve the odds of “herd immunity” for the overall population. “The reason we want to make sure that all of these kids get vaccinated is so we can truly achieve herd immunity. We don’t want to have little pockets of people who might be infectious and not be protected,” said Dr. Georges C. Benjamin, director of the American Public Health Association. The preteen trials were announced Tuesday by Moderna, one of three pharmaceutical companies with vaccines approved for emergency use in adults in the U.S., along with Pfizer-BioNTech and Johnson & Johnson. Moderna and Pfizer vaccines require two doses, while the newer Johnson & Johnson vaccine has a one-dose protocol. The announcement came the same day that the Arizona Department of Health Services announced that just over 1 million Arizonans have been fully vaccinated against the coronavirus. Overall, the state has administered about 2.6 million doses to a little more than 1.6 million people. Moderna CEO Stéphane Bancel said in a statement that more than 53 million doses of his company’s version of the vaccine have been administered in the U.S., but “this pediatric study will help us assess the potential safety and immunogenicity of our COVID-19 vaccine candidate in this important younger age population.” The statement said the new trials would take place in the U.S. and Canada. Dr. Steven Plimpton, the lead investigator for the Phoenix trial, said Tuesday that his office has “already gotten hundreds of calls” from parents interested in getting their children into the trial. He said parents interested in the trial in Phoenix can go to the KidCOVE site for more information or can call 602-368-1928 or 866-913-5454. One University of Arizona expert said it will likely take a little while to get the trials in motion. “I would say sometime in the next several weeks, as they get recruitment on board and they have a critical mass to start with and they have all of the aspects of the trial set up in terms of location, staffing, and everything that they need in place,” said Dr. Shad Marvasti, director of public health and prevention at the University of Arizona College of Medicine. Moderna said that children in the first phase of the trial will receive doses of 25, 50, or 100 micrograms of the vaccine – an adult dose is 100 – depending on their age. Results from that phase will be used to determine dosages in a second phase when come subjects will get a placebo. ad1 Ultimately, Moderna expects to include 6,750 children in the latest trials. “The adult dose for the Moderna is 100 micrograms, but they are starting with 25 micrograms and then basically watching folks and kids to see how they react,” Marvasti said. “If that looks good and there are no major issues, then they will have a group of kids in the study with 50 micrograms and then if that looks okay they will have another group that has 100 micrograms.” He added that Moderna’s trust that the vaccine is safe enough to begin trials on kids could have the added benefit of helping to quell vaccine hesitancy among others. “Hopefully, depending on the results, it will help give people more confidence to get the vaccine, especially if it proves to be as safe and effective in children as it has been in adults,” Marvasti said. The announcement of the preteen trials also comes as the state has ordered schools to begin to resume in-person schooling, after a year in which most students have attended class virtually. Benjamin said that with schools reopening, in Arizona and across the U.S., a vaccine for youth would make a definite difference in controlling the virus, as it would prevent kids from spreading it to each other and then bringing it home with them. Vaccination would also expedite kids’ ability to return to normal. “Getting kids vaccinated, I think, will certainly improve their quality of life and their ability to effectively interact with their friends,” he said. -
 2021-03-21
2021-03-21San Carlos (AZ) to hold a drive-through COVID-19 vaccine clini6c
March 22, 2021 - by News Director Contributed Article SAN CARLOS – The San Carlos Apache Healthcare Corporation is proud to present a COVID-19 vaccine drive-through clinic for SCAT members and their family and friends of the surrounding communities of Globe, Miami, Superior, Hayden, Winkleman, Kearny, Pima, Thatcher, Safford, and Morenci. Our SCAHC Vaccination team will be administering the Pfizer and Moderna vaccine at the San Carlos High School, on Saturday, March 27, from 8 a.m. – 4 p.m. No appointment is necessary. For the Pfizer vaccine, those receiving it must be 16 years of age or older (must have a parent/legal guardian consent if under 18) For the Moderna vaccine, those receiving it must be 18 years of age or older. Please remember to bring your state ID. There is no charge for the vaccine. -
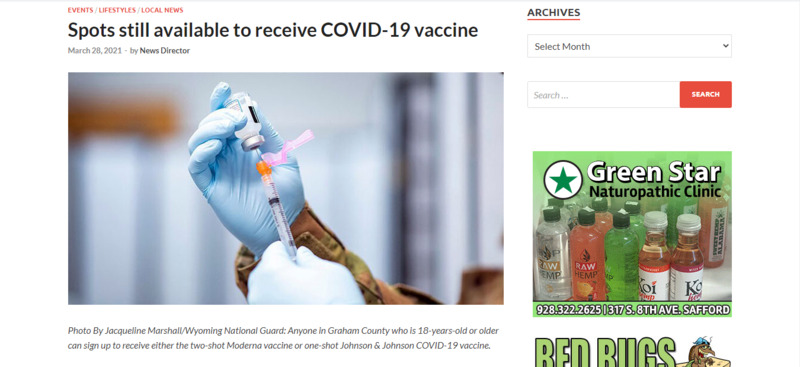 2021-03-28
2021-03-28Graham County (AZ): Spots still available to receive COVID-19 vaccine
Staff Reports SAFFORD – While some in other states attempt to bribe or lie their way to the front of the vaccine lines, that isn’t necessary for those in Arizona. The state has opened its registration to receive a COVID-19 vaccine to anyone 16-years-old and up for the Pfizer vaccine, and anyone 18-years-old and up for the Moderna and Johnson & Johnson vaccines. In Graham County, the Graham County Department of Health and Human Services has advised that there “are many appointments still available” to receive the Moderna vaccine. Anyone 18-years-old and up may schedule an appointment at www.graham.az.gov. The Graham County Health Department Vaccination Center is located in Downtown Safford at 627 W. Main St. In addition to the Moderna vaccine, a vaccination clinic for the one-dose Johnson & Johnson vaccine is also available to any Graham County resident who is 18-years-old or older. A vaccination clinic for the Johnson & Johnson vaccine will be held Friday, April 16. Register for an appointment at www.graham.az.gov. For any additional questions contact the Graham County Department of Health and Human Services at 928-428-0110. -
2021-03-11
Vaccine After Effects
So excited to get my 1st dose of the Moderna vaccine. As a 65 year old, I was eligible early; however, our county's rollout was a HOT mess! The local app didn't work, but I was finally able to secure an appointment with my medical group . . . for a month out. Yikes. Continued research as the days went by, found that RiteAid was offering them. Yay! Was able to book that appointment for only 2 days out! Yippee. Then . . . the day before that appointment, it was cancelled. We Californians are so smug - never thinking that bad weather in other parts of the county affects us. It did! No vaccines available. Rescheduled for a month out. Luckily, our school district was rolling out an in house POD for employees. I jumped on that and was able to get an appointment for the next day, which was the first day for the district. I was sitting on 3 appointments then - and didn't plan to cancel any until I got my shot! Fortunately, all went well and I did get it. (Lucky too because the district had to shut down the next day as well.) So . . . there I was happy. Dose #2 scheduled. Cancelled the other appointments I had. Did have a tiny bit of discomfort the next day, but nothing major. Imagine my surprise when hives (or so I thought) appeared about 10 days later. Did LOTS of research - thank you google - considered that it might be the rash that some experienced after Moderna, but the symptoms progressed. Long story short - not hives - shingles! Even though I did have a shingles vaccine within the past 5 years, I did indeed have shingles. Now - there is no evidence that it is in anyway related to the vaccine, (even found an article that said shingles/vaccine debunked) but I did my duty and reported in on my weekly vsafe/cdc check-in. I am currently on the other side of this and am sure that it will be gone soon. I will always wonder though if there was any relationship. I'm also a tad bit concerned about the after effects of dose #2. I do encourage everyone to participate in the vsafe.cdc.gov follow up. -
 2021-03-05
2021-03-05Vaccinations at the University
This photo shows people lined up to get COVID-19 vaccinations outside the Sun Devil Fitness Complex at Arizona State University in Tempe. My wife received an email from an ASU official sent about 8:00 PM Thursday, March 4 offering university employees access to a distribution of Moderna vaccines at clinics on March 5 and 9. My wife signed up right away and got an appointment for early afternoon on Friday, March 5. I have not been able to get my own appointment through other channels, so I went along with my wife to see if I might be able to get the vaccine. I was turned away, but fortunately my wife now has her first shot and an appointment for the second. It's a start. -
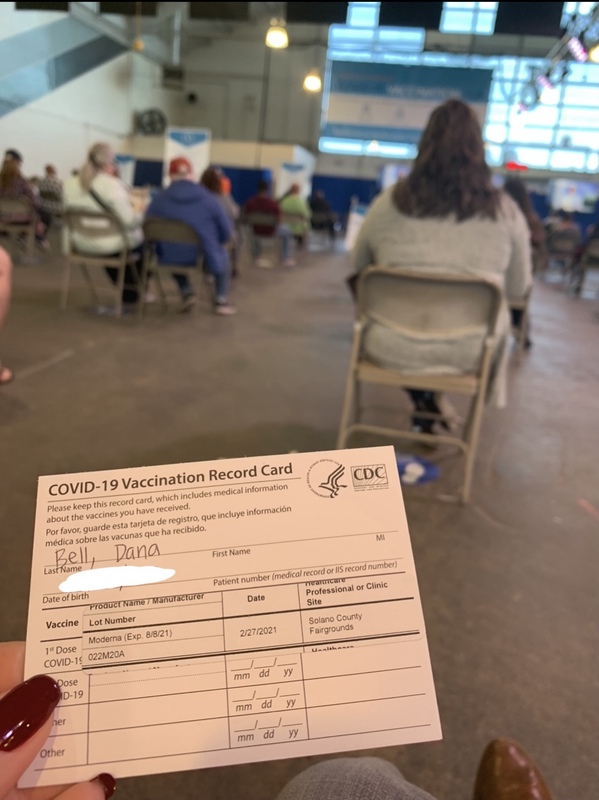 2021-02-27
2021-02-27Finally!!!!!!
What a day! After trying to get an appointment for the Covid vaccine, I was finally able to get a last minute appointment at the fairgrounds. It was a well oiled machine! They were able to vaccinate hundreds of people every hour all day long! I was in and out in 30 minutes. I can’t wait to get back into the classroom and see the faces of the kids I miss so much! Maybe we can find “normal” again? -
 2021-02-26
2021-02-26Health Canada approves AstraZeneca's COVID-19 vaccine
Health Canada has approved the Oxford University-AstraZeneca COVID-19 vaccine, estimating its effectiveness at preventing infection at 62.1%. This means there will now be three vaccines available against COVID-19, the others being Pfizer and Moderna. -
2021-01-25T22:37:00
Sickness Thus far
It is currently the 25 of January 2021 and COVID-19 still runs rampant through the planet. As of now, 99.7 million people have been or are infected with this highly transmissible virus. 2.14 Human beings have died from it, but there is hope. 55 million people have recovered, and the first vaccination has been given to first responders and people over the age of 65. The vaccine was created by a company named Pfizer, Moderna, and AstraZeneca and more improved vaccines are making their way through the lab. It is my prediction that the COVID-19 Pandemic will be over within the next three years. -
 2020-12-23
2020-12-23Vaccine Skeptics
This article explains what is in the vaccine, why it is unique/safe compared to other vaccines, and also explains the side effects of the vaccine. -
2021-01-14
Vaccine Clinic and Personal Vaccine
I work at the Beth Israel Deaconess Medical Center in Boston, MA. My role is usually in education with a background in pre-hospital emergency medicine. Because of my background as a paramedic, I was asked to work in the vaccine clinic for the hospital staff as an observer. My position was to keep an eye on the hospital staff for 15 minutes after they received their Covid vaccine, just in case there were any reactions. Thankfully, my shifts have been very uneventful due to the safety of the vaccine. It was fascinating to talk with the staff when they came back for their second dose, as I was given insight into their experiences with the first dose. This meant that when I went in for my first dose in mid-January, I fully knew what to expect. I received the Moderna vaccine. About four hours after getting the shot, my arm felt quite sore. By the night of the vaccine, my shoulder was throbbing, but it was manageable. For the price of some shoulder pain, the opportunity to receive the shot during the first wave was well worth the discomfort. I get my second dose in early February, so I will see then if I feel as crummy as some of my friends and co-workers have after the second dose. -
2021-01-24
Results from Moderna Vaccine Study
As of today, there have been no deaths attributed to the vaccine and only 10 reported cases of severe reactions to it. -
2021-01-24
Two Main Vaccines and Where to Find Them
1. Pfizer, mRNA vaccine – manufactured by Pfizer and BioNTech, offered across the U.S. in every state 2. Moderna, mRNA vaccine – manufactured by ModernaTX, offered across the U.S. in every state Bibliography: CDC. 2020. “Coronavirus Disease 2019 (COVID-19).” Centers for Disease Control and Prevention. February 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Fabout-vaccines%2Fhow-they-work.html. Calgary, Open. n.d. “COVID-19 Vaccine Distribution Allocations by Jurisdiction - Pfizer | Data | Centers for Disease Control and Prevention.” Data.cdc.gov. Accessed January 25, 2021. https://data.cdc.gov/Vaccinations/COVID-19-Vaccine-Distribution-Allocations-by-Juris/saz5-9hgg. -
2021-01-24
Differences and Similarities Between the COVID-19 Vaccines
There are 3 types of vaccines that are or will be available in the U.S. 1. mRNA: uses part of the COVID-19 virus to create proteins in our bodies that our immune system can recognize and remember in order to fight the virus 2. Protein subunit: has pieces of the proteins that the COVID-19 vaccine uses (not the actual virus) that the body will recognize in the future that do not belong in the body 3. Vector: injection of a weakened but live virus that has the genetic material that causes COVID-19 (a vector virus) that will cause the body to make the proteins that cause COVID-19 and force the immune system to remember that protein and fight it in the future The two being offered across the U.S. right now are both mRNA vaccines that require 2 shots 21 days apart Both vaccines are tested with a 95% effectivity, but that effectivity is only proven to be true after both doses are administered and there is not substantial long-term effect research yet Bibliography: CDC. 2020. “Coronavirus Disease 2019 (COVID-19).” Centers for Disease Control and Prevention. February 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Fabout-vaccines%2Fhow-they-work.html. “Covid-19 Vaccine Frequently Asked Questions.” 2021. New England Journal of Medicine. 2021. https://www.nejm.org/covid-vaccine/faq?cid=DM108101_&bid=351587577. -
 2021-01-20
2021-01-20Vaccine and the Community
Somebody who doesn’t work in the medical field might view this as a not so complicated process. But somebody planning how to distribute the vaccine to everyone in the community sees many challenges. Both Moderna and Pfizer vaccines require a second dose in a specific time period. One challenge presents itself with the homeless population, how to get them back for their second dose and within the required time period. Suggestions for solving this problem include giving free transportation and meals for the people receiving their vaccine, but there seems to be no easy answer. Some experts are trying to find a solution by educating and informing the public, hoping to ease fear and get people in for both doses. -
 2021-01-14
2021-01-14I got the vaccine!
On Thursday, January 14, I got the first round of the Moderna vaccine. I am a high school teacher in American Falls Idaho. I think I might be one of the first teachers in the country to get the vaccine. I am very excited and honored to be among the first. I’m very grateful to my school district for working hard to get vaccines. -
 2021-01-05
2021-01-05NPR News Report on Vaccine & Malfunctioning Refrigerator in CA
Reading this on the NPR (National Public Radio) News Application -
 12/10/2020
12/10/2020Moderna vaccine to be available to 75% of 'eligible population' in N.W.T in early 2021
The Northwest Territories government expects the Moderna vaccine for COVID-19 to be available to 75 per cent of the territory's "eligible population" in "early 2021," according to a media advisory issued Thursday afternoon. -
 2020-12-04
2020-12-04U.S. Surgeon General: 'We Are Absolutely Ready' To Distribute COVID-19 Vaccine
The US Surgeon General talks about the upcoming distribution of the COVID-19 vaccine, case spikes, and travel over Thanksgiving. -
 2020-11-18
2020-11-18"The End of the Pandemic Is Now in Sight" - The Atlantic Monthly
With the development of two viable COVID-19 vaccines, it appears that the end of the pandemic appears to be at hand in the near future. In an article for the Atlantic Monthly magazine, journalist Sarah Zhang explains how these viable vaccines were developed using new technologies and how the resolution of the pandemic is now more dependent on policy choices made by political leaders, namely the President of the United States. During the initial months of the COVID-19 pandemic, medical professionals, epidemiologists, and vaccinologists were in the dark about the symptoms, treatability, and curability of the disease. After months of intense hands-on experience and in-depth genomic research, the companies Pfizer and Moderna have developed viable vaccine candidates. But these vaccines are different from typical vaccines: they are mRNA vaccines. This means that they work by injecting mRNA which encodes viral proteins, rather than injecting a weakened or dead SARS-CoV-2 virus. mRNA vaccines, according to Zhang, were once thought to be potentially unviable, but the positive preliminary results of the Pfizer and Moderna mRNA vaccines may mark the beginning of a new era of vaccine research and development. In the future, Zhang says, mRNA vaccines may be developed for the Zika virus or for personalized forms of cancer. However, a major drawback of mRNA vaccines is their fragility, as they require extremely cold temperatures to be preserved. Now that these vaccines may be available for public use in the near future, it is up to the United States' political leadership to formulate policies to promote the vaccination of the populace and the mitigation of COVID-19 infections during the winter. According to Zhang, "Every infection we prevent now—through masking and social distancing—is an infection that can, eventually, be prevented forever through vaccines." -
 2020-11-17
2020-11-17新型コロナワクチン開発 米モデルナ「94.5%効果」(2020年11月17日) - Development of new corona vaccine US Moderna "94.5% effect" (November 17, 2020)
アメリカのバイオテクノロジー企業「モデルナ」は開発中の新型コロナウイルスのワクチンについて、「94.5%の効果が得られた」とする臨床試験の暫定的な結果を発表しました。 モデルナは16日、ワクチン開発の最終段階となる大規模な臨床試験の結果を発表しました。新型コロナウイルスに対して「ワクチンが94.5%の確率で効果を示した」としています。アメリカではファイザー社のワクチンでも「90%以上」の有効性が確認されていて、来月中にも両社のワクチンの緊急使用が始まる可能性が出てきました。ただ、このタイプのワクチンは低温で保存・運搬する必要があるなど課題も残されています。日本政府はモデルナと2500万人分のワクチン供給を受ける契約を結んでいます。 American biotechnology company "Moderna" has announced the preliminary results of a clinical trial that "94.5% of the effect was obtained" for the new coronavirus vaccine under development. On the 16th, Moderna announced the results of a large-scale clinical trial, which is the final stage of vaccine development. "The vaccine has a 94.5% chance of being effective against the new coronavirus," he said. In the United States, Pfizer's vaccine has been confirmed to be "90% or more" effective, and it is possible the emergency use of both companies' vaccines will begin by the end of next month. However, there are still issues with this type of vaccine, such as the need to store and transport it at low temperatures. The Japanese government has a contract with Moderna to receive vaccines for 25 million people. Video translated by Youngbin Noh
