Items
Tag is exactly
vaccination
-
2023-05-19
Griffith Observatory
The Griffith Observatory is located in Los Angeles, California, where COVID-19 restrictions were lifted in February 2023 statewide. This picture was taken in May of 2023, just a few months after the Observatory stopped asking for proof of vaccination before letting you in and lifted its face mask mandate. I had been to the Observatory several times before the pandemic, but I stopped once the pandemic hit. Although the Observatory was open during the pandemic, it required visitors to wear face masks and show proof of vaccination before allowing them to enter the facility. -
 2021-09-16
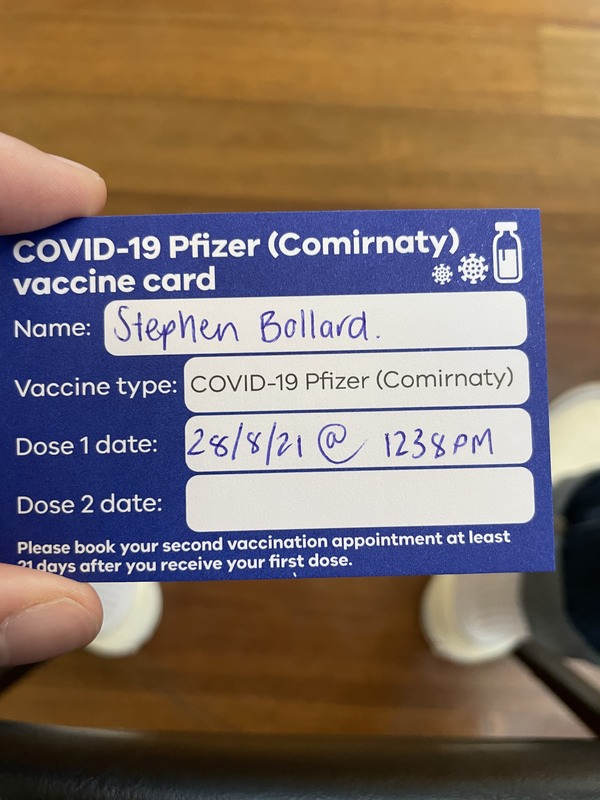
2021-09-16Hotel California & Casa Bonita
My spouse and I love road trips and concerts. Covid definitely slowed us down! In the summer of 2021, we decided to take a road trip culminating in a concert in Denver, Colorado. Which concert? The Eagles - Hotel California! We drove and camped through Idaho, Wyoming, Utah, and Colorado. In Denver, we toured the famous restaurant Casa Bonita and went to the concert. For the concert, everyone needed a vaccination card and masks were required (although not widely worn). It was fun and felt a little like back-to-normal. We had a great time! -
 2022-03
2022-03School Trip to New York COVID-19
As a school, we take a trip to New York and Boston every other year. We had planned to take a trip in October 2021 but many of the venues in New York were still closed so we had to postpone until spring 2022. Even then, there were still a lot of students who did not want to get the vaccine that was required to enter all indoor venues at the time. We had to split the trip so those without vaccinations could go later when the mandate was lifted. There were still mask mandates and we had to present our vaccination cards at every venue. Despite the restrictions though, we had a great time in New York. -
 2022-06-12
2022-06-12Eurostar station in London, June 2022
During my honeymoon, my husband and I were in London, Summer of 2022. We had spent two weeks there and it was time to take the Eurostar train through the Channel Tunnel to Amsterdam. What particularly struck me was how serious the ticket agents and French government officials (the train must enter through France) were about Covid-19 Vaccine Record Cards. If someone did not have their official government-issued Covid vaccine certificate, they were absolutely not permitted on the train. It was very serious and made me anxious, even though I held on to mine tightly, along with my passport. We waited in the entry line for 40 minuets to an hour to get to the check point, the guards were very intense and scrutinized every passenger, and we eventually entered the train on to our destination. This was the first trip we had taken since the pandemic began, and by this time Europe and parts of Asia were opening back up for tourism. Many people we saw in London and Amsterdam at this time were no longer wearing masks, and since my husband and I were vaccinated, neither did we. We had an amazing honeymoon and I am grateful that the pandemic was slowing down and the countries we visited were accepting tourists. -
 12/01/2021
12/01/2021Jillian Schemenauer Oral History, 2021/12/01
Jillian Schemenauer was born and raised in Chippewa Falls, Wisconsin. From there she went on the receive her bachelor’s degree and master’s degree from the University of Minnesota-Mankato. She is now working on her Doctorate degree at UW-Milwaukee. In her interview, she discusses moving to Milwaukee in the early days of the Covid-19 pandemic. She also discusses the increase in mental illnesses in her students and colleagues and the reasons behind the increase. -
 12/10/2021
12/10/2021Shae Havner Oral History, 2021/12/10
In this interview, Shae Havner discusses her experiences as a mental health therapist during the pandemic and the changes in her career and her clients. She talks about how the pandemic affects mental health, both positively and negatively, and the rise in domestic abuse cases. She also gives insight into how COVID-19 affected her home life as a mother and how the pandemic has affected her sons as well as what her family and friends did to have fun during the shutdown. She lives in Fall Creek, Wisconsin, and works in Eau Claire, Wisconsin, and compares how the two cities responded to the pandemic. She also brings up vaccinations, the booster shot, and getting her children vaccinated. -
 2023-03-15
2023-03-15Kit Heintzman Oral History, 2023/03/15
Kit Heintzman is a recovering academic currently residing in Lenapehoking, who was trained in the medical humanities with a special interest in queer theory, animals, and the history of nationalism. Kit has developed a singular collection of oral histories of the pandemic for A Journal of the Plague Year, collected from a range of individuals with widely diverse experiences. That collection addresses significant silences surrounding the pandemic broadly and within JOTPY more narrowly. In this item Kit is interviewed by Angelica and Erin, both with Arizona State University, about Kits collection process. -
 2021-08-28
2021-08-28The First Jab
HIST30060: This is an image of when I was waiting to leave the Royal Exhibition Building following my first vaccination. The experience was not something I was unfamiliar with, throughout high school I received regular vaccinations, the only downside this time was there not being a bowl of lollies to reward myself with as there was during high school. I had been anticipating the worst of symptoms after what I had heard from others, but fortunately all I really got was a stiff arm. This was also amidst the beginnings of the anti-vax movement and protests that we unfortunately are so accustomed to at this point. -
 2022-07-05
2022-07-05Masks optional for fully-vaccinated customers
This is a sign I found outside a shoe store at Arizona Mills Mall. It says that masks are optional for fully-vaccinated customers. I didn't go in the store, so I don't know if they would check for vaccination status or not. From my experience with other places with similar signs, no employee has asked about my vaccination status before shopping. I could see this being enforced a year ago, but not now. -
 2022-04-07
2022-04-07Arrival Requirement at Pago Pago International Airport for April 7 Hawaiian Air Flight
The Governor and Lt. Governor of American Samoa has issued arrival requirements for travelers on the April 7th Hawaiian Air Flight coming into the island. These requirements are to ensure the safety of the people of American Samoa and as well as the travelers traveling to American Samoa from COVID-19. -
 2022-05-04
2022-05-04Erika Groudle Oral History, 2022/05/04
Erika Groudle is a resident of Monroe, Washington. She lives in a tiny house with her partner on her mother’s property. In this oral history interview Erika discusses working with kids during the pandemic and her opinion on how they handle mask wearing. Additionally, Erika discusses her “pandemic garden,” caring for her grandfather, staying connected to friends and family during the pandemic, how she first realized the pandemic was close to home, and the realities of living in a state that not only had the first case and death of COVID-19 in the United States of America, but also highly publicized protests in Seattle. Interviewer: Jason Inskeep Interviewee: Erika Groudle -
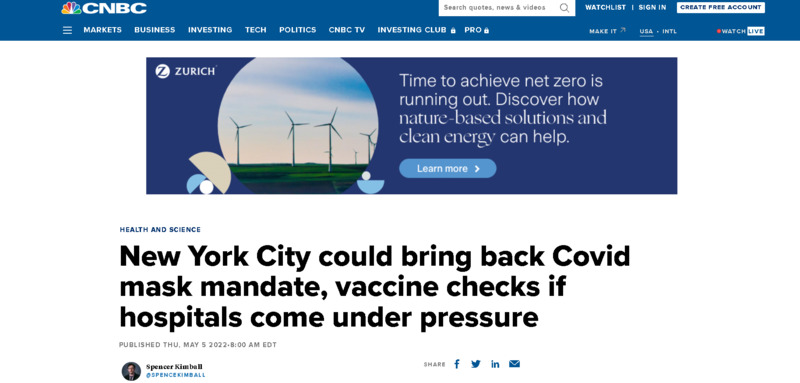 2022-05-05
2022-05-05New York City could bring back Covid mask mandate, vaccine checks if hospitals come under pressure
This is a news story from CNBC News by Spencer Kimball. New York City might bring back the mask mandate and vaccine checks if hospitals become too overwhelmed. New York City increased its COVID alert level from low to medium earlier this week as infections have kept on rising. Health Commissioner Ashwin Vasan said New York might reinstate mandatory masking and vaccine checks if the city raises its Covid alert level to high. New York's alert system is based off of CDC guidance and hospital protocols. Mayor Eric Adams ended mandatory vaccine checks at restaurants and other indoor venues in March, in addition to the mask mandate for people attending school. Masks are still required on buses, rail, and on subways in New York City. New York City, as of right now, has 80% of their population fully vaccinated. -
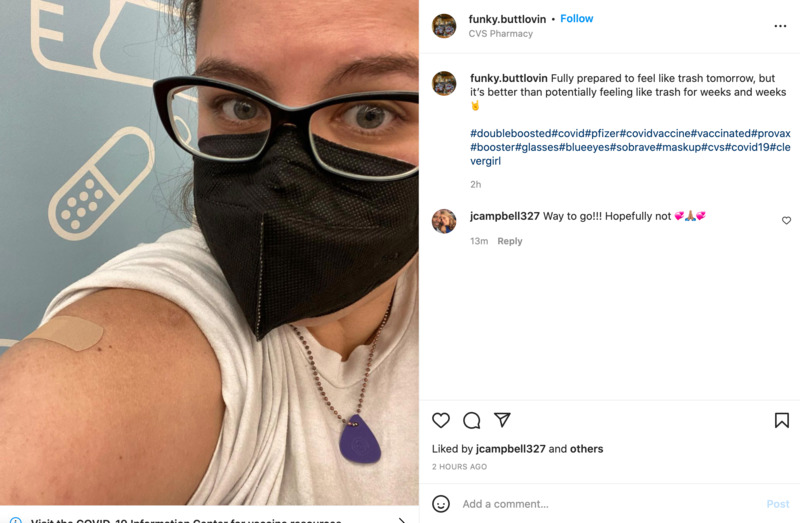 2022-04-29
2022-04-29Double Boosted
This is an Instagram post by funky.buttlovin. This person received their two extra doses of the COVID vaccine. People taking selfies has been a common trend on social media after having received a COVID vaccine. -
 2022-04-25
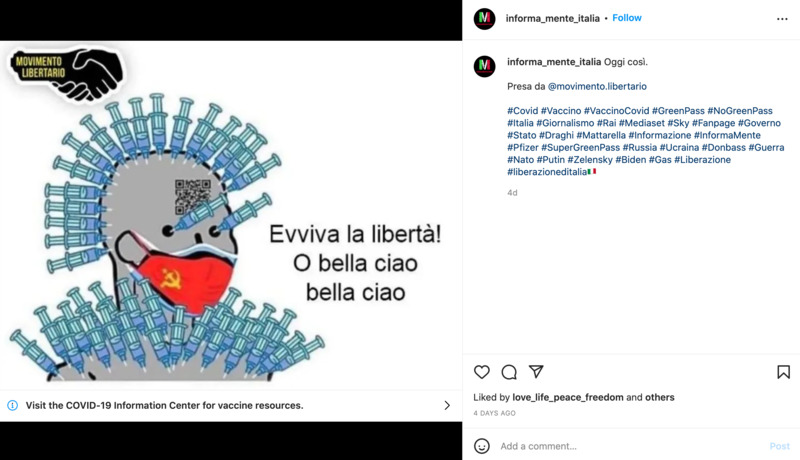
2022-04-25Covid Green Pass
This is an Instagram post by informa_mente_italia. It is criticizing the use of the Green Pass in Italy, where vaccination status is checked at certain places. The picture shown is making fun of the people that get the vaccines, and there are many needles to show that they are never ending, due to booster shots. This is a political issue within Europe at the moment because the Green Pass for COVID controls movement of people and what parts of society they are allowed to participate in. -
 2022-04-13
2022-04-13American Samoa COVID Cases Situational Report #22
This is the twentieth-second report released by the American Samoa Department of Public Health regarding the rise of covid cases in American Samoa. As of April 13, 2022, positive cases have risen to 5457 from 5254 on April 08, 2022. A total of four individuals are hospitalized, and nineteen recorded deaths related to COVID-19 have been documented. American Samo's vaccination coverage of individuals who are fully vaccinated currently stands at 83.7%. -
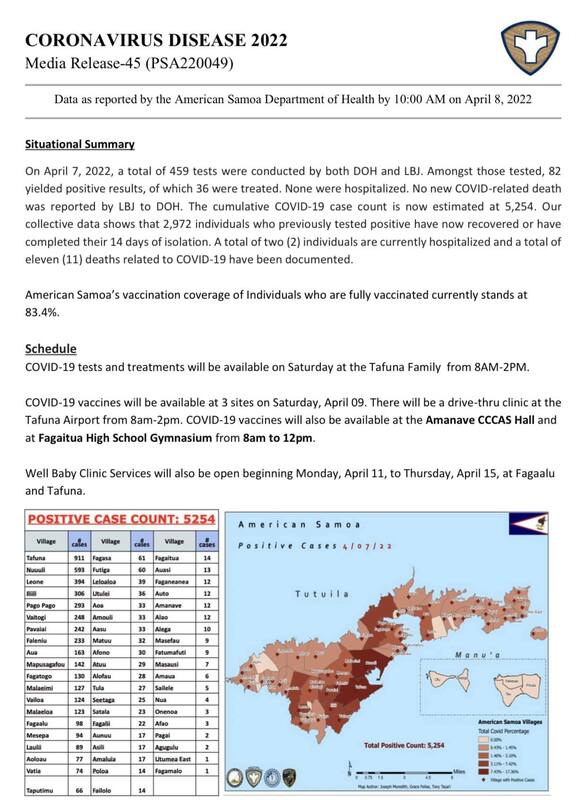 2022-04-08
2022-04-08American Samoa COVID Cases Situational Report #21
This is the twentieth-first report released by the American Samoa Department of Public Health regarding the rise of covid cases in American Samoa. As of April 08, 2022, positive cases have risen to 5254 from 4957 on April 05, 2022. A total of two individuals are hospitalized, and eleven recorded deaths related to COVID-19 have been documented. American Samo's vaccination coverage of individuals who are fully vaccinated currently stands at 83.4%. -
 2022-04-05
2022-04-05American Samoa COVID Cases Situational Report #20
This is the twentieth report released by the American Samoa Department of Public Health regarding the rise of covid cases in American Samoa. As of April 05, 2022, positive cases have risen to 4957 from 3756 on March 29, 2022. A total of five individuals are hospitalized, and eight recorded deaths related to COVID-19 have been documented. -
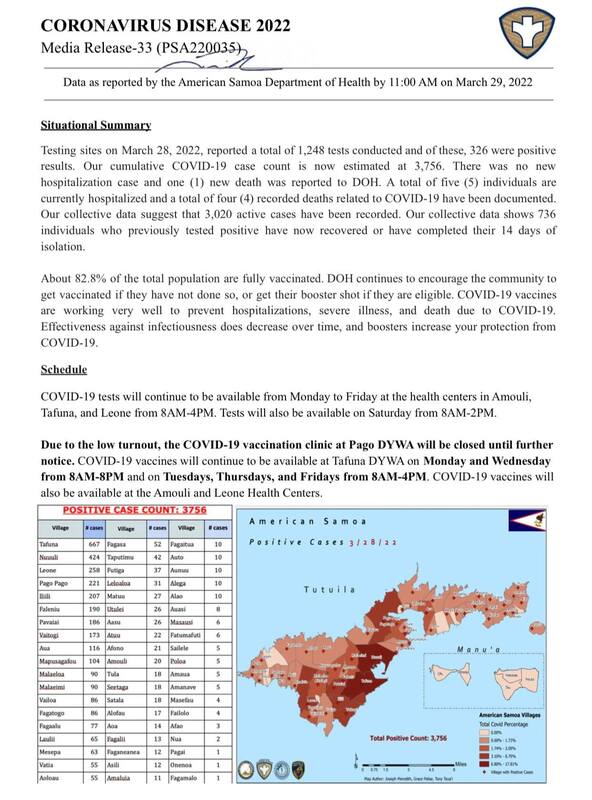 2022-03-29
2022-03-29American Samoa COVID Cases Situational Report #19
This is the nineteenth report released by the American Samoa Department of Public Health regarding the rise of covid cases in American Samoa. As of March 29, 2022, positive cases have risen to 3756 from 3381 on March 27, 2022. A total of five individuals are hospitalized and 4 recorded deaths related to COVID-19 have been documented. -
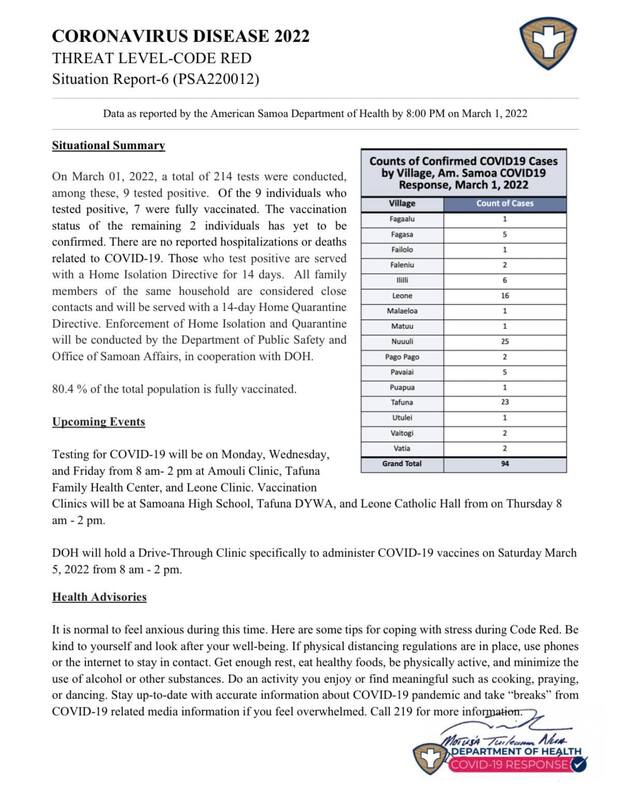 2022-03-01
2022-03-01American Samoa COVID Cases Situational Report #5
This is the fifth report released by the American Samoa Department of Public Health in regards to the rise of covid cases in American Samoa. As of March 1, 2022, positive cases have risen to 94 from 85 on February 28, 2022. -
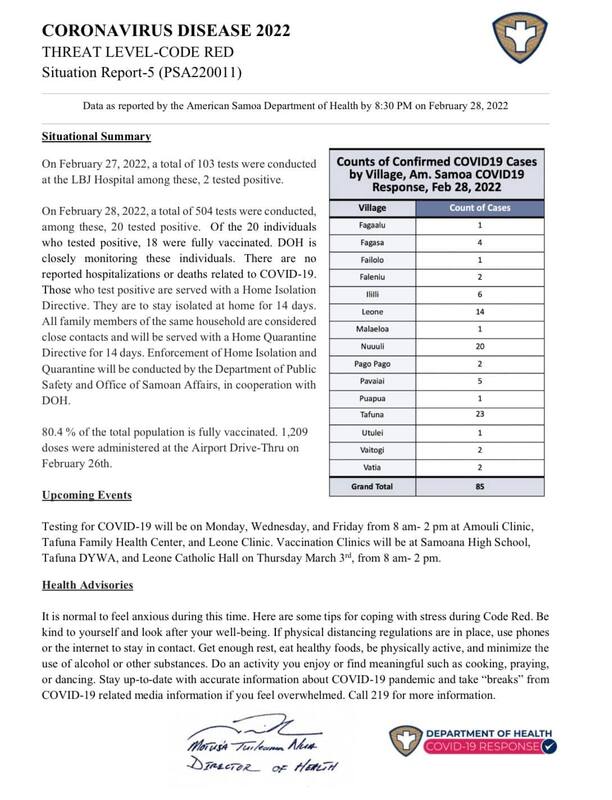 2022-02-28
2022-02-28American Samoa COVID Cases Situational Report #4
This is the fourth report released to the public by the Department of Public Health notifying the public of American Samoa of the number of cases that the island has as of February 27, 2022. From the past three reports, it seems as if from the past three days till now, the number of cases jumped from 22 to 85. While a summary is provided of the situation, upcoming events and health advisories are also included in the report for the public to continue to practice and where to get tested, vaccinated, or the booster shot. -
 2022-04-06
2022-04-06Fully Vaccinated
This is an Instagram post by lynobtena. It shows a picture of a kid that had just received his second dose of Pfizer. There is a place for kids to get their picture taken after getting vaccinated, with cardboard cutouts of a Paw Patrol character and Iron Man. -
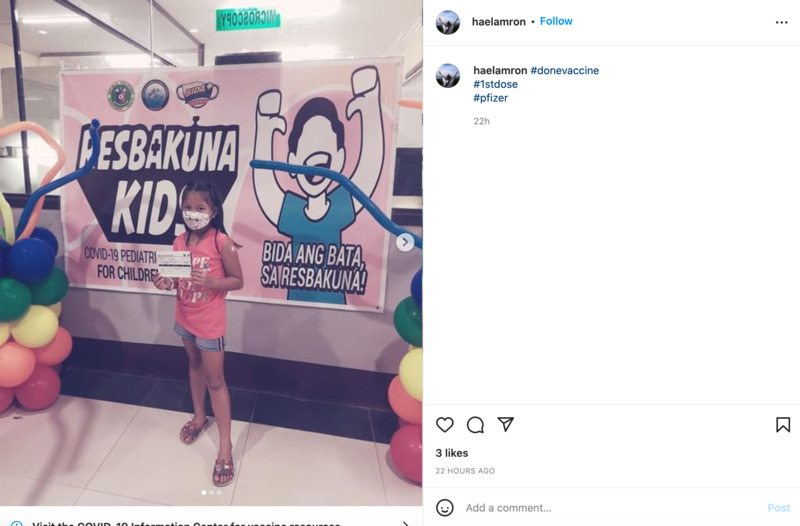 2022-04-07
2022-04-07The First Dose
This is an Instagram post by haelmron. This shows a picture of a little kid that had just received their first Pfizer dose. It looks like a special place was set up for kids to get their picture taken after getting vaccinated. -
 11/12/2020
11/12/2020Kristine Benusa Oral History, 2020/11/12
-
 2021-12-11
2021-12-11Olivia Valenti Oral History, 2021/12/11
It illustrates what college was like during the pandemic -
 2020
2020New England Student in COVID
It seems as though every winter all of the kids in schools get a cold. Classrooms have a chorus of sniffles and coughs until springtime and we all suffer sickness together. At least, that’s how it started. My college sent an email to all students, staff, and faculty, saying the school would be monitoring the COVID-19 situation in other countries on February 10th, 2020 and there was no threat to worry about. Everyone left for spring break on March 8th, 2020, expecting to be back in a week. Instead, we got an “extra week” of the break to make sure anyone who traveled could quarantine, just in case. That week turned into a handful more and started online classes ASAP. Students were given the opportunity to go back to the college in a 3-hour window to retrieve any materials necessary for a few weeks online until the surge dies down. Fortunately, I am studying computer science, so a majority of my professors had minimal difficulty making the change, but others were not as fortunate. Quickly, the handful of weeks became the remainder of the semester. All courses would be graded on the basis of pass/fail if the students elected for each individual course they were enrolled in, due to the nature of this huge and unprecedented turnaround. All exams were online, many professors canceled their midterms to alleviate stress from the students and fears of cheating. We would receive semi-weekly updates from the college, mostly fluff pieces about missing the student body with information that was important sprinkled in. Eventually, we were permitted to sign up for a window of time to go and move our belongings out of the dorms, once the state allowed outside travelers in. In the midst of all of the chaos, I transferred colleges and started the next academic year attending one that was much larger and had more resources at its disposal to deal with COVID-19. This school had planned to welcome students back to campus in fall 2020 with a few expectations in place. They had devised a “COVID-19 Compliance” system to keep the population safe and maintain records of who was following protocol. Students would have a “green badge” assigned to them in the morning if: they had completed a daily symptom check-in that was negative, they were up-to-date on their twice-weekly COVID tests and had not been marked as a close contact to someone who had tested positive. Had one of these not been completed, you would have a yellow badge to mark non-compliance, a red badge for isolation, or an orange badge if you were symptomatic. Students must show a green badge to enter ANY campus building. Some classes were online, others hybrid in-person/online at the discretion of the professors. Masks were to be worn at all times, students must get vaccinated once they were eligible, dining areas were to-go only, the campus was littered with signs to promote 6 feet of social distancing, and a student-run campaign called “F*ck It Won’t Cut It” was started to bring attention to the urgency of staying compliant to stay on campus. We would receive weekly updates about the status of the campus’s overall positivity rate. It felt like a shell of a college experience, as students could not visit other students’ residences, no clubs could have in-person meetings, attendance at sporting events was prohibited, and students reporting other students for non-compliance created an atmosphere of disdain. We are now in the second full academic year of the pandemic and there are a few deviations from what I described for fall 2020. Now, COVID tests are once weekly rather than twice, students can now visit other residences and attend sporting events, all of the dining spaces have opened up to sit-in dining, masks are still required at all times, all classes are in person, and the “F*ck It Won’t Cut It” campaign has been retired. It seems as though we are creeping towards the idea of a “typical” college experience, but it feels like this will have an everlasting impact on the next few incoming classes of students and change college as people know it. -
 2021-12-01
2021-12-01Ana Suarez Oral History, 2021/12/01
I interviewed a student that attends St. Mary’s University and is a work study in the Law School. I wanted to get her voice out and get an idea of what her perspective was on this ongoing pandemic. Hearing her and speak about where she was when the pandemic struck really reminded me that we all faced the same problems and that no one knew what the outcome was going to be. As a student in college, I’m sure that it was just as hard to know that schools would be shutting down and having no clue as to what the next step would be. Going back home and attending class virtue was hard especially if some did not have the resources for online fees, or laptops, etc. Hearing from Ana, and knowing that she struggled financially while in quarantine and making ends meet really makes us think that everyone had it hard. But in the long run she was able to go back to school and received the vaccination and made sure she followed all the policies that were in effect at the University. At least make it feel like some things were back to normal. -
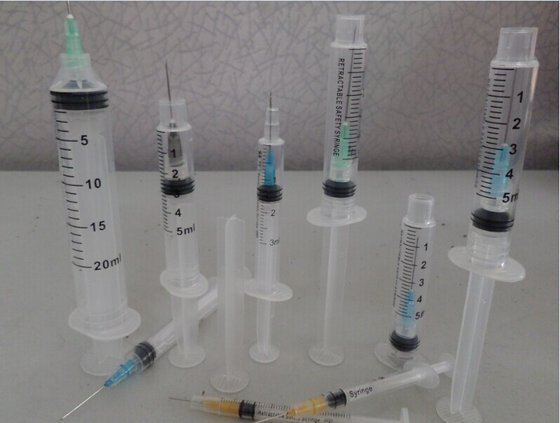 2021-11-29
2021-11-29Needle stick accommodations made available to the public
Not everyone is a fan of hypodermic needles ( personally I am not). Healthcare has made considerable accommodations for those who are available for vaccinations by changing the size of the needle gauge; child and adult. By the healthcare industry developing new, different, and special accommodations for providing vaccinations via needle sticks are taking into consideration not everyone likes needles. For the most the public have a fear of needles. To now make the vaccinations a lot more welcoming for you as child /adult have options. There are now even needle-less "sticks" -
 2021-11-22
2021-11-22First-year resident assistant: Covid Campus
It is obvious that the covid-19 pandemic has changed the college experience for all students. However, what was it like for those who do not know a pre-covid college experience. For some students, all they know is a covid campus. For Amanda Swan, a first-year resident assistant, her unique experience and the pandemic have allowed her to better relate to her residents. Having experienced a senior year of high school online and isolated gave makes allows her to better understand residents who have had similar experiences. Many residents who have not been on campus or have not been given the opportunity to experience a pre-covid college semester have been left to readjust to more social life. On top of many responsibilities of a resident assistant and academic duties, Amanda Swan is a very involved student navigating her way through college. Despite being her first time as a resident assistant and her first time living on campus, Amanda Swan has been able to serve as a resource for residents at St. Mary’s University. -
 2021-10-01
2021-10-01My Breakthrough COVID-19 Case
October 1, 2021, I woke up with a mild headache and a stuffy nose. I didn't think much of it—I had started drinking coffee again and needed a cup, and I’m mildly allergic to my own cats. My headache went away after I had my coffee, and my congestion ceased after I took my Claritin. After working remotely and basically not socializing for all of 2020 and up through August 2021, I was happy to be out and doing things again. In August, I started working and attending class in-person again, as well as spending time with friends. I still masked up and washed my hands according to guidelines, but it did seem like standards for that were slipping. I take public transit most days, and I’d seen a number of people who either weren’t wearing masks or not wearing them properly. But I still thought I was fairly safe since I followed COVID-19 recommendations, was fully vaccinated, and my campus has an extremely high vaccination rate (100% of students are vaccinated or have exemptions, and 98% of faculty/staff). So when I woke up experiencing what I thought were symptoms of seasonal allergies, I didn’t think anything of it. I went on a date that afternoon, and then out for drinks with friends later that night. I was very tired when I went home that night, but I chalked it up to how I’d over-committed myself in the initial euphoria of being able to participate in things again. Besides, I was sleeping better than I’d slept in years. The next day, my congestion was worse and I was coughing. I had an intermittent headache, but I assumed it was just a cold. One of my classmates that I sit next to had had one recently, and she’d tested negative for COVID, so I just assumed I’d picked it up from her. I remained congested and feeling gross that weekend, enough to call out from my shift on Sunday out of an abundance of caution, but I figured I’d be ready to be back by the time I had class and work again on Wednesday. But Monday afternoon I was working on some of my reading and realized I couldn’t smell the new (and very strong) candle in my living room. To test whether it was just the candle or whether it was me, I sniffed my perfume and finally even put peppermint essential oil right under my nose, and...nothing. Figuring that it was likely I had COVID at this point, I scheduled a test for the next day. I felt bad about having to get there—was it better to take an Uber or a train/bus? Which was safer for everyone involved? I ended up taking a Lyft, but I left the windows down and made sure I had cough drops so I wouldn’t cough. Once I arrived at the testing center (where I was the only patient), they got me through quickly and told me they’d be doing PCR testing and I could expect my results within a couple days. I called out of work for the week and let my professors know I likely had it. I woke up on Thursday morning to see my results had arrived, and I had tested positive. I called my school for contact tracing, and they notified the classmates I sit next to and my coworkers. I texted my friends I’d been out with Friday night and the person I went out with, and it was strange to feel almost ashamed. I had behaved responsibly, but I still felt as though I’d done something wrong in contracting COVID. And I was exhausted, tired of coughing, and just wanted my mom. I continued to improve, and I felt mostly better by the time my isolation period ended on the 11th. My sense of smell had started to come back, so I wasn’t as worried about a permanent loss there. I was a little concerned by the disregard for no-contact delivery I’d requested when getting food/groceries, but it had mostly been okay. My shifts at work had been given away, even though I was better and out of isolation by then. On the bright side, my cats were thrilled to have had me home that much, so at least it was a good experience for someone. Everyone I notified directly or via contact tracing tested negative, fortunately. When I started going back to things, I just wanted to scream on the train when I saw people not wearing masks or wearing them improperly. I still do, especially as the number of cases rises. -
 2021-05-25
2021-05-25Breakthrough COVID-19 cases possible but rarity shows vaccine effectiveness
This graphic shows the rates of infection and hospitalization for breakthrough infections for COVID-19 among vaccinated people (as of May 2021) -
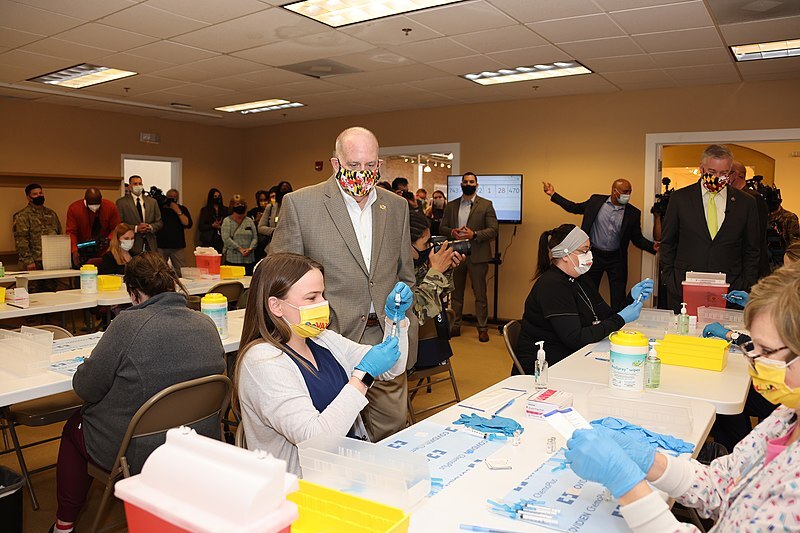 2021-03-26
2021-03-26Hagerstown Mass Vaccination Site
This photo shows a number of people at a mass vaccination event. -
 2021-03-26
2021-03-26Participating in a Mass Vaccination Event
I was excited to get my vaccine as soon as the FDA approved the Pfizer vaccine. However, as a fairly healthy person who worked remotely, I was by no means going to be the first wave of vaccination. Truthfully, I thought I wouldn’t have a chance to get vaccinated until June or so, and I resigned myself to staying inside. In early March, I got an email from my school—the United Center was hosting a mass vaccination event, and they had more doses than the original target groups could use. I hurried to sign up. It filled quickly; I had a few friends tell me they were unable to get in. I was lucky, and I went to get my first dose near the end of March. Supposedly, Uber was offering free rides to/from the United Center (up to a certain amount, at least) for those seeking to get vaccinated. However, I kept getting error messages, so I made my way there by other methods. I panicked since I was almost late to my appointment for the first dose, but my worries faded when I arrived. The clinic volunteers kept the roped off lines going quickly and smoothly, though everyone was kept at least six feet apart. Once you’d been fully signed in—you showed your ID, your appointment voucher, got your temperature taken, and were issued an information packet—you waited to be sent to one of the FEMA people doing the vaccinating. I was called and got my first dose over with quickly and without any fuss, and then I was sent off to the tent where you waited to make sure you didn’t have any adverse side effects within the first 20 minutes. I was fine, so I went home with my vaccination card and instructions to return in 3 weeks. I returned 3 weeks later (in mid-April), and it went even more smoothly! They had worked out even more kinks, and everyone seemed relieved. While I’d been tired and a little sick a couple days after the first dose, the second one presented no problems. Later, I learned that a few of my friends were not only also part of the United Center mass vaccination event, but were there on the same days! I didn’t see them, but I’m not surprised given the efficiency of the process. Over the summer, the United Center’s vaccination program closed after it slowed significantly. So while I will be getting my booster shot soon, it won’t be as part of a mass vaccination endeavor. I’m a little reticent, simply because I don’t know what to expect from going to a pharmacy for it! -
 2021-02-26
2021-02-26Illinois to open federal mass vaccination site at United Center
Parking lots of the United Center will soon host a new mass vaccination site for Illinoisans. Gov. JB Pritzker says the site will have the capacity to give 6,000 doses of vaccine per day. The home of the Chicago Bulls and Blackhawks will open as a vaccination site on March 10. But, construction is already underway. This will be one of the several community vaccination centers led by the Biden administration. Doses will come directly from the federal government instead of taking vaccine away from the allotment for the state and the city of Chicago. Leaders explained seniors will have exclusive access to appointments before the site officially opens. However, FEMA hasn’t set dates for those appointments at this time. Reporters asked how Pritzker could guarantee this facility would create easier access for those in need compared to wealthy Chicagoans. “In the city of Chicago, in Cook County, and across the state, we’ve all made and are continuing to make efforts to attract people of color to people who are most vulnerable to making those appointments, giving them access wherever we can. Having a site in a location like the United Center makes it more easily accessible,” Pritzker emphasized. Chicago Mayor Lori Lightfoot said rideshare service Uber will provide 20,000 free rides to help people get to the site. Information about scheduling appointments for vaccinations should become available in the coming days. “With this new site, we’ll now be able to take our vaccination success to a whole new level and bring to bear the historic and inclusive recovery that is soon to come,” Lightfoot explained. Getting Black and brown residents vaccinated Still, the state has a significant issue getting Black and Latinx Illinoisans vaccinated. U.S. Sen. Dick Durbin explained a recent study showed minority neighborhoods in Chicago had a vaccination rate of 5%. The majority-white areas of Chicago currently report 13% of the population vaccinated. Durbin says the United Center site should help. “The faster we can get people vaccinated, the more quickly we can escape the grip that this pandemic has had on our nation for so long, the less likely we’re gonna see mutations and variations which we have to fight in different ways,” Durbin added. The Springfield native said the federal government could provide more help with vaccine distribution bypassing the American Rescue Plan. President Joe Biden has asked Congress to approve the $1.9 trillion package with specific portions going to mass vaccination sites and $1,400 stimulus checks. Pritzker noted things are getting better in the long battle with COVID-19. “Someday not too far from now, we’ll be at the United Center not for a life-saving shot, but for a game-winning shot,” Pritzker said. -
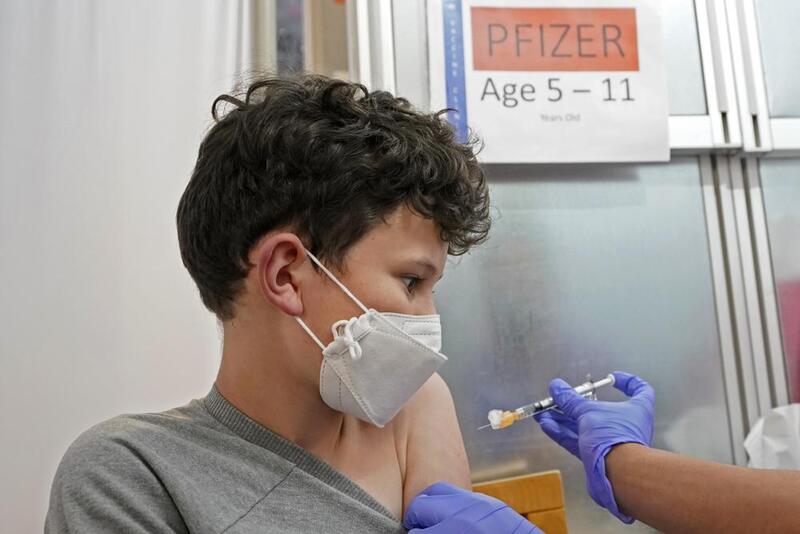 2021-11-10
2021-11-10Children Ages 5-11 Now Eligible for Vaccine
The campaign to vaccinate elementary school age children in the U.S. is off to a strong start, health officials said Wednesday, but experts say there are signs that it will be difficult to sustain the initial momentum. About 900,000 kids aged 5 to 11 will have received their first dose of the COVID-19 vaccine in their first week of eligibility, the White House said, providing the first glimpse at the pace of the school-aged vaccination campaign. “We’re off to a very strong start,” said White House COVID-19 coordinator Jeff Zients, during a briefing with reporters. Final clearance for the shots was granted by federal regulators on Nov. 2, with the first doses to kids beginning in some locations the following day. The estimated increase in vaccinations in elementary school age children appears similar to a jump seen in May, when adolescents ages 12 to 15 became eligible for shots. Now nearly 20,000 pharmacies, clinics and physicians’ offices are offering the doses to younger kids, and the Biden administration estimates that by the end of Wednesday more than 900,000 of the kid doses will have been given. On top of that, about 700,000 first-shot appointments are scheduled for the coming days. About 28 million 5 to 11 year-olds are now eligible for the low-dose Pfizer vaccine. Kids who get their first of two shots by the end of next week will be fully vaccinated by Christmas. The administration is encouraging schools to host vaccine clinics on site to make it even easier for kids to get shots. The White House is also asking schools to share information from “trusted messengers” like doctors and public health officials to combat misinformation around the vaccines. A initial surge in demand for vaccinations was expected from parents who have been waiting for the chance to protect their younger kids, especially before the holidays. About 3% of newly eligible children in the U.S. got first shots in the first week, but the rate of vaccinations in varied widely around the country, as it has for adult vaccines. California Health and Human Services Secretary Dr. Mark Ghaly said Wednesday that more than 110,000 Californians ages 5 to 11 have received their first coronavirus shot — 9% of kids that age in the state. “We are starting to see this pick up and I’m really encouraged about what this means for our state,” Ghaly said. On the other ends of the spectrum, Idaho reported just 2,257 first shots, or 1.3% of the newly eligible kids there. In West Virginia’s Cabell County, high demand led local health officials to start setting up vaccination clinics in all the county’s public middle schools. A spokeswoman for the county health department said there were some lines for vaccines in the first few days after the doses were approved for kids ages 5 to 11, but that things have slowed since then. Some experts say that nationally, demand could also begin to recede soon. They note polling data suggests only a fraction of parents have planned to get their kids shots immediately, and they suspect the trend will play out like it did earlier this year when kids ages 12 to 15 were first able to get shots. In the first week after vaccines for that age group were authorized in May, the number of adolescents getting a first shot jumped by roughly 900,000, according to an American Academy of Pediatrics review of federal data. The next week, it rose even further, to 1.6 million. “There was an initial burst,” said Shannon Stokley of the Centers for Disease Control and Prevention. But then the number dropped steadily for months, interrupted only briefly in early August as the delta variant surged and parents prepared to send children back to school. Adolescent vaccinations have since flagged considerably, to just 32,000 getting their first shots last week. Only about half of adolescents ages 12 to 17 are fully vaccinated, compared to 70% of adults. It’s unlikely that vaccination rates in young kids will be as high as what’s seen in adults — or even in adolescents, some experts said, unless they are required for school. Part of the reason is that adults are far more likely than children to suffer serious illness or die from COVID-19, they noted. “Parents may have the perception it may not be as serious in young children or they don’t transmit it,” said Stokley, the acting deputy director of the CDC’s Immunization Services Division. But more than 2 million COVID cases have been reported in U.S. children ages 5 to 11 since the pandemic started, including 66 deaths over the past year, according to CDC data. “We’re going to have a lot of work to do to communicate to parents about why it’s important to get children vaccinated,” she said. Zients said the effort to vaccinate younger kids is still ramping up, with new clinics coming on line. Government officials expect the number of children who are vaccinated to keep rising in the days and weeks ahead, he said. “We are just getting started,” he said. Earlier this year the White House set — and missed — a July 4 goal to have at least certain percentage of U.S. adults vaccinated. Officials have not announced a similar target for kids. Dr. Lee Savio Beers, president of the American Academy of Pediatrics, called the new numbers reassuring and said the rollout appears to be going smoothly for the most part. She noted however that with a lower dose and different vials than for older kids, the rollout requires more steps and that some states have been slower in getting vaccine to providers. Initial data from some areas show Black children lagging behind whites in getting their first doses, which Beers said raises concerns. “It’s really important to make sure the vaccine is easily accessible in a wide variety of places,” Beers said. -
 2021-09-16
2021-09-16The Importance of Context in Covid-19 Vaccine Safety
Vaccine safety is critical for the successful implementation of any vaccination program, especially during a pandemic. In February 1976, the Centers for Disease Control and Prevention confirmed a cluster of cases of severe influenza-like illness among Army recruits at Fort Dix, New Jersey.1 A swine influenza A strain that resembled the 1918 pandemic influenza strain was identified,2 and a vaccination program was subsequently initiated for the entire U.S. population. After more than 40 million persons were vaccinated, a small excess risk of Guillain–Barré syndrome was noted, with an attributable risk of approximately 1 case per 100,000 doses administered. Given these concerns and because the pandemic did not materialize, the vaccination program was halted in December 1976 so that the issue could be explored further. This experience shed light on the need for real-time vaccine safety surveillance and the importance of context in decision making during a pandemic. In a study now reported in the Journal by Barda et al., the investigators simultaneously evaluated the risk of adverse events among persons (≥16 years of age) who had received the BNT162b2 vaccine (Pfizer–BioNTech) and the risk of the same events after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.3 The authors used data from the largest integrated payer–provider health care organization in Israel, in conjunction with data on SARS-CoV-2 polymerase-chain-reaction tests and data on coronavirus disease 2019 (Covid-19) vaccine administration from the Israeli Ministry of Health. This use of multiple data sets highlights the importance of investment in digital capabilities and meaningful integration across systems in order to provide real-time answers to key public health questions. The design of rigorous postauthorization vaccine safety studies during the Covid-19 pandemic has been a challenge because the pandemic itself has caused changes in health care utilization, the rollout of Covid-19 vaccines has occurred in phases because of initial supply limitations, and there have been disparities in access to vaccines. Barda et al. broadly addressed many of these challenges by emulating a trial that matched eligible vaccinees to unvaccinated controls according to sociodemographic characteristics, the number of preexisting chronic health conditions, previous health care utilization, and pregnancy status. In the vaccination analysis, the study included 42 days of follow-up (i.e., 21 days after the first dose and 21 days after the second dose). This analysis accounted for seasonal and secular trends by matching on the day of vaccination, rather than relying on historical risk estimates that may not have been comparable in the pandemic setting. In the SARS-CoV-2 analysis, a similar approach was used to match persons with a newly diagnosed infection to uninfected persons. Although the risk estimates in the vaccination and the SARS-CoV-2 analyses were not directly comparable because of differences in the populations (i.e., events were evaluated per 100,000 vaccinated persons and per 100,000 infected persons, respectively), these risks were placed in context. The most salient example is myocarditis, which has received much attention recently given the preponderance of reported cases after vaccination among adolescents and young adults and the incidence of myocarditis observed after SARS-CoV-2 infection.4-6 In the population-based cohort in the study conducted by Barda and colleagues, the risk ratios for myocarditis were 3.24 (95% confidence interval [CI], 1.55 to 12.44) after vaccination and 18.28 (95% CI, 3.95 to 25.12) after SARS-CoV-2 infection, with risk differences of 2.7 events per 100,000 persons (95% CI, 1.0 to 4.6) and 11.0 events per 100,000 persons (95% CI, 5.6 to 15.8), respectively. What is even more compelling about these data is the substantial protective effect of vaccines with respect to adverse events such as acute kidney injury, intracranial hemorrhage, and anemia, probably because infection was prevented. Furthermore, the persons with SARS-CoV-2 infection appeared to be at substantially higher risk for arrhythmia, myocardial infarction, deep-vein thrombosis, pulmonary embolism, pericarditis, intracerebral hemorrhage, and thrombocytopenia than those who received the BNT162b2 vaccine. National discussions about benefit–risk balance often focus on the benefits of preventing symptomatic disease, hospitalization, or death due to Covid-19 and the risks of serious adverse events after vaccination.7,8 As specific adverse events such as myocarditis are highlighted, however, the lack of corresponding specificity about benefits can hamper efforts to communicate effectively with patients. Messenger RNA (mRNA) vaccines may be associated with myocarditis, but they can also prevent cases of myocarditis, acute kidney injury, arrhythmia, and thromboembolic disease. The key to comparing these risks depends on the risk of SARS-CoV-2 infection to an individual person, and that risk can vary according to place and over time. Given the current state of the global pandemic, however, the risk of exposure to SARS-CoV-2 appears to be inevitable. One major limitation of this study is the lack of risk estimates according to age group and sex. For example, thrombosis with thrombocytopenia syndrome occurs predominantly in young adult women who have received adenoviral vector vaccines against SARS-CoV-2, whereas myocarditis predominantly occurs in male teens and young men who have received mRNA vaccines.5,9,10 Age- and sex-stratified comparisons that reflect local epidemiologic factors might support public understanding of different approaches to vaccine use in different countries, such as Israel, the United Kingdom, and the United States. Other limitations of the study include the paucity of data regarding younger teens and children, the conservative assumption that vaccines have no effect on transmission, and the absence of medical record review to validate computable phenotypes (i.e., algorithms used to identify a cohort on the basis of patient records). As new knowledge of the safety and benefits of vaccines continues to evolve, studies like this one may help to support decision making about the use of Covid-19 vaccines. The benefit–risk balance should be reassessed, refined, and communicated as the disease burden changes, new variants and safety signals emerge, and vaccine effectiveness begins to wane. Context matters, which means that we as a country need to be ready for continual learning and change. -
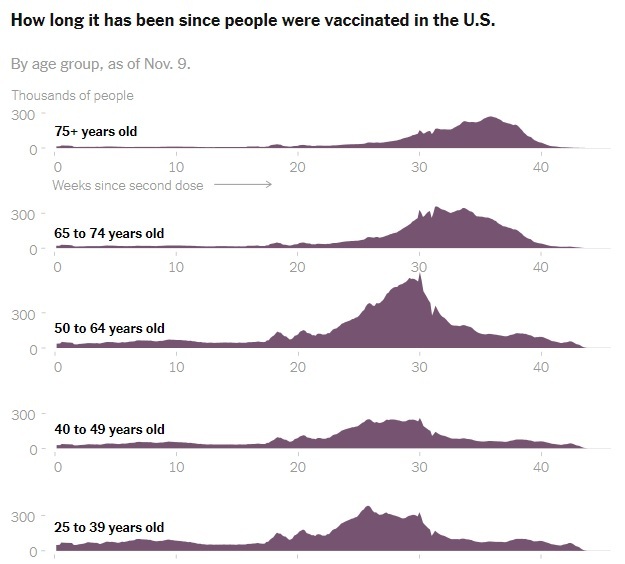 2021-11-11
2021-11-11What We Know So Far About Waning Vaccine Effectiveness
As tens of millions who are eligible in the United States consider signing up for a Covid-19 booster shot, a growing body of early global research shows that the vaccines authorized in the United States remain highly protective against the disease’s worst outcomes over time, with some exceptions among older people and those with weakened immune systems. But while the vaccines’ effectiveness against severe disease and hospitalization has mostly held steady, even through the summer surge of the highly transmissible Delta variant, a number of published studies show that their protection against infection, with or without symptoms, has fallen. Public health experts say this decline does not mean that the vaccines are not working. In fact, many studies show that the vaccines remain more than 50 percent effective at preventing infection, the level that all Covid vaccines had to meet or exceed to be authorized by the Food and Drug Administration back in 2020. But the significance of these declines in effectiveness — and whether they suggest all adults should be eligible for a booster shot — is still up for debate. -
 2021-04-06
2021-04-06Biden will offer a virus update as the pace of vaccination accelerates
President Biden will promote his administration’s success in accelerating the pace of coronavirus vaccinations during two appearances on Tuesday, as officials in nearly every state say they will make shots available to all adults by his target of April 19. Three months into his presidency, Mr. Biden confronts an escalating migrant surge at the border with Mexico and has embarked on a grind-it-out effort to ram through a $2.3 trillion infrastructure bill. But the virus remains his primary focus. And he wants the country to know that — so he is offering multiple updates each week, along with helpful visual cues, like standing next to a giant Easter bunny wearing a mask. On Tuesday afternoon, Mr. Biden will travel to Alexandria, Va., to tour a vaccination site at the Virginia Theological Seminary. Later, at the White House, he will deliver remarks emphasizing recent successes, including the milestone of delivering four million vaccinations in one day over the weekend. More than three million doses are now being given on average each day, compared with well under one million when Mr. Biden took office in January, according to the Centers for Disease Control and Prevention. Every state has now given at least one dose to a quarter or more of its population. About 62.4 million people — 19 percent of Americans — have been fully vaccinated. On Monday, Gov. Larry Hogan of Maryland announced that all Maryland residents 16 or older would be eligible from Tuesday for a shot at the state’s mass vaccination sites, and starting April 19 at any vaccine provider in the state. Also on Monday, Gov. Philip D. Murphy of New Jersey and Mayor Muriel Bowser of Washington, D.C., said residents 16 or older would be eligible on April 19. Gov. Kate Brown of Oregon announced Tuesday that all Oregonians over the age of 16 were eligible to receive a vaccine. The state had been limiting the doses to those with underlying conditions and frontline workers. That leaves one state, Hawaii, keeping to Mr. Biden’s original deadline of May 1. In Hawaii, 34 percent of residents have received at least one dose. Alabama has vaccinated the lowest proportion of its residents, at 25 percent. Along with dangerous coronavirus variants that were identified in Britain, South Africa and Brazil, new mutations have continued to pop up in the United States, from California to New York to Oregon. The shots will eventually win, scientists say, but because each infection gives the coronavirus a chance to evolve further, vaccinations must proceed as quickly as possible. For now, however, cases are rising sharply in parts of the country, with some states offering a stark reminder that the pandemic is far from over. Yet again, governors across the country have lifted precautions like mask mandates and capacity limits on businesses. -
 2021-01-19
2021-01-19Incoming CDC Director to Prioritize Communication, COVID-19 Vaccine Rollout
As Rochelle Walensky, MD, MPH, prepares to assume the role of CDC director on January 20, the former professor of medicine at Harvard Medical School and infectious disease physician at Massachusetts General Hospital and Brigham Women’s Hospital faces a myriad of challenges wrought by the ongoing coronavirus disease 2019 (COVID-19) pandemic. January 21st marks the 1-year mark since the first case of COVID-19 was reported in the United States, while current data indicate the country has surpassed 400,000 deaths. In comparison, the 1918 flu pandemic took 675,000 American lives, while the US reported a total of 405,000 fatalities during World War II. Even at the unprecedented speed with which pharmaceutical companies have developed vaccines for COVID-19, rollout has been fragmented at the state level while racial disparities in administration rates are beginning to become apparent. In an effort to improve the national rollout of COVID-19 vaccines, Walensky plans to increase the CDC’s communication to combat any hesitancy in receiving the vaccine, and indicated she wanted to increase media appearances above those made by current director Robert Redfield, MD, who departs with any remaining Trump administration officials Wednesday. She said making sure science-based communication is effectively disseminated to the public in layman’s terms is a top priority. “Science is now conveyed through Twitter. Science is conveyed on social media, on podcasts, and in many different ways. And I think that's critical,” Walensky said during a livestreamed interview with JAMA's Howard Bauchner, MD, the journal's editor-in-chief. When confronting vaccine hesitancy or anti-vaxxer sentiment on social media, “There's just this massive void and the right information, I think, is not getting out there… I want to make sure that the science is conveyed. We have to say it to one another. We have to say it to the public. And then we have to say it in other forms.” Internally, Walensky hopes to bolster the voices of scientists already employed by the CDC. Under the Trump presidency, “they have been diminished. I think they've been muzzled,” Walensky said. “This top tier agency—world renowned—hasn't really been appreciated over the last 4 years, and really markedly over the last year. So I have to fix that.” Although some states have been widely successful in administering the allotment of COVID-19 vaccines they were given, many have reported roadblocks. Part of the Biden administration’s plan to enhance rollout is to expand vaccine allocation to 4 key locations: federally qualified health centers, community vaccination centers (ie, stadiums), mobile units, and pharmacies. “Part of the challenge with COVID-19 was that we had a frail public health infrastructure to start. It wasn't ready to tackle what it was given,” Walensky said. As director, she hopes to bring this reality to Congress’ attention. “We're in this because we had warnings for many, many other public health scares in the last 20 years and we didn't fix our public health infrastructure and our data infrastructure,” in response to those tests. In order to meet President-elect Biden’s goal of 100 million vaccinations in 100 days, the constraints currently faced by federal and state governments need to be mitigated. “We have to titrate our supply and our eligibility so that we somehow hit the sweet spot, wherever it is we are, with how much supply we have and how many people are eligible,” Walensky said. While the CDC set the initial guidelines for vaccine eligibility and revised them this month, the Trump administration left actual rules and distribution processes to states, resulting in wide variation across the country. Some states adopted stricter standards that led to the waste of vaccines, while loose adherence has led to long lines and confused residents. Expanding the population of those eligible to administer the vaccine can also help alleviate these roadblocks. These individuals can include retirees, the Public Health Commissioned Corps, medical military, upper level medical and nursing students, dentists and veterinarians. Increasing both the number of vaccination sites and vaccinators will also help address the equity problems brought to light by the pandemic. “We want to make sure that we can deliver volume, but also volume to the people in places that might be harder to reach.” In a collaborative approach, the federal government will step in at a state-by-state level and offer help based on each state’s unique challenges, Walensky said. -
 2020-12-31
2020-12-31Trump administration falls far short of vaccination goals as new virus variant looms
Logistical problems at the heart of the federal government’s faltering rollout of coronavirus vaccines came into sharper view Thursday as the Trump administration fell vastly short of its goal of delivering an initial shot to 20 million people by the end of December. On the final day of a bleak year, only about 2.8 million people had received the shot, according to the Centers for Disease Control and Prevention — the first of two doses needed to provide immunity to the virus. Around 14 million doses had been distributed as of Wednesday, according to Gustave Perna, chief operating officer of Operation Warp Speed, and a total of 20 million doses have been allocated. Though the figures are an underestimate — data collection on vaccinations has lagged — the doses administered so far represent just a small fraction of the ambitious targets outlined by officials from the administration’s Operation Warp Speed program in the fall. “We’d have liked to have seen it run smoothly and have 20 million doses in to people today, by the end of 2020, which was the projection,” Anthony S. Fauci, the government’s leading infectious-disease expert, said in an interview with NBC’s “Today” show on Thursday. “Obviously it didn’t happen, and that’s disappointing.” Nationwide, states and health-care providers continued to grapple with unpredictable timelines for when new vaccine shipments would arrive and in what quantities, while chronically underfunded public health departments struggled to muster the resources to carry out mass injections of front-line workers and vulnerable people. Fauci said that he hoped momentum for vaccinations would build in the first weeks of the new year and bring the country closer to its immunization goals. “But there really has to be more effort in the sense of resources for the locals, namely the states, the cities, the counties, the places where the vaccine is actually going into the arms of individuals,” he said. “We have to support the local groups, the states and the cities to help them get this task done, which is a very prodigious task.” Under the Trump administration’s plan, the federal government supplies vaccines to states but leaves it to state officials to prioritize residents, send doses to providers and get shots into people’s arms. The approach — as well as a litany of logistical problems — has caused a varied distribution effort. Local health departments and hospitals tasked with administering the vaccines have complained that they do not know when shipments will come or if they will receive additional resources, said Oscar Alleyne, and epidemiologist and chief of programs and services for the National Association of County and City Health Officials, which is made up of about 3,000 local health departments. “Some health departments have only received vaccines as recently as this week,” Alleyne told The Washington Post. “I had one health department that told me they had received their vaccines the day after Christmas.” Alleyne compared the communication concerns to those that cropped up during the H1N1 pandemic in 2009, when unclear guidance hampered efforts to get the population vaccinated. “It really boils down to ensuring a very transparent process,” Alleyne said. “There will always be a lag between the doses allocated and those shipped; between those shipped and those administered; and between those administered and those reported to CDC as administered,” Michael J. Pratt, a spokesperson for Operation Warp Speed, said in a statement. “We’re working to make those lags as small as possible.” At the Texas Medical Center, the largest medical complex in the world, the approach has already created logistical challenges. Hospital officials on the campus in south Houston often don’t know exactly when to expect new shipments or precisely how many vials they’ll receive, according to Bill McKeon, the center’s chief executive officer. That leaves the center with just a couple days’ worth of vaccine inventory on hand at a time, he said. “At best, we hear estimates. It’s a day-to-day situation,” McKeon told The Post. “We hear that we may be getting more next week but we’re not sure.” To date, the center has administered the first of the two injections to about 60,000 people, averaging more than 4,000 a day, according to McKeon. That includes some of the center’s 120,000 employees, as well as patients with underlying conditions who are first in line for inoculation. But it’s only a tiny portion of the sprawling metropolitan area the center serves. Until hospital officials can better predict how many vaccine doses they’ll have available week after week, McKeon said, vaccinating more people, faster, will be an uphill battle. “You can’t do scheduling with a couple days of inventory. We wouldn’t put a patient through the process of coming to the hospital, leaving their home, and then say, ‘Sorry we don’t have the inventory,’ ” he said. “We can’t be bold and just say, ‘Let’s do ten thousand a day.’ ” McKeon called on the federal government to take a more active role, possibly offering more large-scale vaccination centers, and relieve pressure on state officials, whom he said were “rowing in the same direction” as providers. There will be a growing need not just for more health-care workers to give the shots, he said, but for people who can perform the administrative work of calling patients, verifying their personal information and signing them up for injections. “I’m not seeing the grand strategies on a national basis, and I’m concerned, because this is a war,” he said. “Every day that we delay on some of those grander strategies we’re going to see losses of life.” As the distribution of vaccines has proceeded in fits and starts, coronavirus deaths and hospitalizations have soared to new heights. More than 125,000 people around the country were in hospital beds battling covid-19, the disease caused by the virus. Hospitalizations have exceeded 100,000 since Dec. 2. The nation on Wednesday also recorded a record 3,862 deaths in a day. The previous record, set on Dec. 17, was 3,406. New daily reported cases were trending upward again, after dipping during the week of Christmas. Family gatherings and spikes in holiday travel make it all but certain that the new year will bring yet another wave of infections. Compounding fears about the accelerating virus spread, a new, more transmissible variant of the coronavirus has cropped up in multiple U.S. states after circulating in the United Kingdom. The presence of the mutated pathogen only added to the need for vaccinations to ramp up quickly, said Scott Gottlieb, former Food and Drug Administration commissioner. “The Covid vaccine could be a tool to help reduce the impact of current wave of epidemic spread,” he tweeted Thursday. “But we’re largely missing the narrow window we had to deploy it rapidly enough to alter the present trajectory of death and disease in January. The new variant makes this more urgent.” Clarification: This story has been updated to cite Operation Warp Speed’s distribution numbers. It has also been updated to note that Operation Warp Speed has allocated 20 million vaccine doses to states. -
 2020-12-14
2020-12-14The U.S. Approves a Vaccine
The Food and Drug Administration authorized Pfizer’s Covid-19 vaccine for emergency use on Friday, clearing the way for millions of highly vulnerable people to begin receiving inoculations within days. The authorization is a turning point in a pandemic that has taken more than 290,000 lives in the United States. With the decision, the United States becomes the sixth country — in addition to Britain, Bahrain, Canada, Saudi Arabia and Mexico — to clear the vaccine. Today, we ask the science and health reporter Donald G. McNeil Jr. what might happen next. -
 2020-09-14
2020-09-14Pfizer and BioNTech announce plan to expand Covid-19 vaccine trial
Pfizer and BioNTech are moving to enlarge the Phase 3 trial of their Covid-19 vaccine by 50%, which could allow the companies to collect more safety and efficacy data and to increase the diversity of the study’s participants. The companies said in a press release that they would increase the size of the study to 44,000 participants, up from an initial recruitment goal of 30,000 individuals. The U.S. Food and Drug Administration will have to approve the change before it goes into effect. “The companies continue to expect that a conclusive readout on efficacy is likely by the end of October,” the press release said. The Pfizer and BioNTech study is likely to be among the first in the U.S. to report efficacy data from a Phase 3 trial. Related: AstraZeneca resumes Covid-19 vaccine trials in the U.K. Expanding the trial will likely make it easier for the company to demonstrate whether the vaccine is effective against SARS-CoV-2, the virus that causes Covid-19. The companies also said that the change will allow the study to include a more diverse population. The companies said the study will now include adolescents as young as 16, people with stable HIV, and those with hepatitis C or hepatitis B. The companies said that the trial is expected to reach its initial target of 30,000 patients next week. Moderna, which started its trial on the same day as Pfizer, said on Sept. 4 that it is working to increase the diversity of trial participants in its study, “even if those efforts impact the speed of enrollment.” Related: Covid-19 Drugs and Vaccines Tracker The Pfizer/BioNTech study could finish sooner than Moderna’s, even though the two began on the same day, for other reasons, as well. Both vaccines require a second shot; Pfizer’s is given after three weeks, while Moderna’s is given after four. The Pfizer trial also starts to count cases of Covid-19 sooner after participants receive their shots than the Moderna study. But the Pfizer/BioNTech vaccine could also prove to be one of the most difficult of the experimental vaccines to distribute, should they prove effective. The vaccine must be kept at a temperature of -70 degrees Celsius. There has been political pressure to move a vaccine quickly, with President Trump saying that one could be available before election day. Last week, several drugmakers, including Pfizer, issued a pledge not to move a vaccine forward sooner than was justified by the results of their clinical trials. -
 2020-07-14
2020-07-14Moderna Phase 1 results show coronavirus vaccine safe, induces immune response
Moderna Inc’s experimental vaccine for COVID-19 showed it was safe and provoked immune responses in all 45 healthy volunteers in an ongoing early-stage study, U.S. researchers reported on Tuesday. Volunteers who got two doses of the vaccine had high levels of virus-killing antibodies that exceeded the average levels seen in people who had recovered from COVID-19, the team reported in the New England Journal of Medicine. No study volunteers experienced a serious side effect, but more than half reported mild or moderate reactions such as fatigue, headache, chills, muscle aches or pain at the injection site. These were more likely to occur after the second dose and in people who got the highest dose. Experts say a vaccine is needed to put an end to the coronavirus pandemic that has sickened millions and caused nearly 575,000 deaths worldwide. Moderna was the first to start human testing of a vaccine for the novel coronavirus on March 16, 66 days after the genetic sequence of the virus was released. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, whose researchers developed Moderna’s vaccine candidate, called the results “good news,” noting that the study found no serious adverse events and the vaccine produced “reasonably high” levels of virus-killing or neutralizing antibodies. “If your vaccine can induce a response comparable with natural infection, that’s a winner,” Fauci said in a telephone interview. “That’s why we’re very pleased by the results.” Moderna shares jumped more than 15% in after-hours trading on Tuesday. The U.S. government is supporting Moderna’s vaccine with nearly half a billion dollars and has chosen it as one of the first to enter large-scale human trials. A successful vaccine could be a turning point for Cambridge, Massachusetts-based Moderna, which has never had a licensed product. Moderna’s shot, mRNA-1273, uses ribonucleic acid (RNA) - a chemical messenger that contains instructions for making proteins. When injected into people, the vaccine instructs cells to make proteins that mimic the outer surface of the coronavirus, which the body recognizes as a foreign invader, and mounts an immune response against. The results released Tuesday involved three doses of the vaccine, tested in groups of 15 volunteers aged 18-55 who got two shots, 28 days apart. The groups tested 25, 100 or 250 micrograms of the vaccine. Adverse events after the second dose occurred in seven of the 13 volunteers who got the 25-microgram dose, all 15 participants who received the 100 microgram dose and all 14 who got the 250 microgram dose. In the highest-dose group, three patients had severe reactions such as fever, chills, headache or nausea. One of these had a fever of 103.28 Fahrenheit (39.6 C). “We didn’t see any events that are characterized as serious adverse events,” said lead author Dr Lisa Jackson of Kaiser Permanente Washington Health Research Institute in Seattle, referring to reactions that require hospitalization or result in death. In June, Moderna said it selected the 100-microgram dose for its late-stage study to minimize adverse reactions. At that dose, Moderna said the company is on track to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, starting in 2021, from the company’s internal U.S. manufacturing site and strategic collaboration with Swiss drugmaker Lonza. “It’s a good first step,” said Dr William Schaffner, a vaccine expert at Vanderbilt University Medical Center who was not involved in the study. “There’s nothing here that would inhibit one from going ahead to the Phase 2/Phase 3 trials,” he said. In April, Moderna expanded the Phase 1 trial to include adults over 55, who are more at risk of serious disease, with the aim of enrolling 120 volunteers. Moderna said it will follow study volunteers for a year to look for side effects and check how long immunity lasts. Moderna started its phase 2 trial in May and expects to start a phase 3 trial on July 27. Phase 1 trials aim to ensure a treatment is safe and help determine an effective dose. Phase 2 trials test a treatment in a larger group and get an early read on effectiveness. Phase 3 trials are conducted in a large group of individuals to confirm efficacy and identify rare side effects. Moderna’s Phase 3 trial will be conducted in 30,000 volunteers. -
 2020-05-21
2020-05-21HHS, AstraZeneca Speed COVID-19 Vaccine Development; First Doses Due in October
The Trump administration announced early today that HHS and AstraZeneca will collaborate on a coronavirus disease vaccine called AZD1222. A statement released from HHS said the partnership will make “at least 300 million doses” of the vaccine available, “with the first doses delivered as early as October 2020.” The vaccine is one originally developed at the University of Oxford; the university and AstraZeneca announced a global development agreement for the vaccine on April 30. In its own statement early today, AstraZeneca said a phase 1/2 clinical trial of the vaccine began last month to assess its safety immunogenicity and efficacy in over 1000 healthy volunteers, who are 18 to 55 years of age. These volunteers are all in the United Kingdom. Late-stage trials would begin in several countries based on these results, the statement said. According to HHS, the agreement between AstraZeneca and the Biomedical Advanced Research and Development Authority (BARDA), an agency within HHS, would essentially kick start manufacturing of the doses while phase 3 clinical studies are under way this summer, involving 30,000 volunteers in the United States. BARDA will spend up to $1.2 billion for research, technology transfer, and scaled-up manufacturing, Emergency use authorization or licensure from FDA would be needed for the vaccine to reach the public, the statement said. As for the timeline, “Early milestones enable BARDA and AstraZeneca to determine how the program progresses forward.” “This contract with AstraZeneca is a major milestone in Operation Warp Speed’s work toward a safe, effective, widely available vaccine by 2021,” said HHS Secretary Alex Azar. “Getting a vaccine to the American public as soon as possible is one part of President Trump’s multi-faceted strategy for safely reopening our country and bringing life back to normal, which is essential to Americans’ physical and mental well-being in so many ways.” “The Trump Administration is making multiple major investments in developing and manufacturing promising vaccines long before they’re approved so that a successful vaccine will reach the American people without a day wasted,” Azar said. Besides the BARDA agreement, AstraZeneca said it has reached deals with the Coalition for Epidemic Preparedness Innovations (CEPI), the Vaccine Alliance and the World Health Organisation (WHO), to ensure the fair allocation and distribution of the vaccine around the world. AstraZeneca is also in discussions the Serum Institute of India and other potential partners to boost production and distribution. AstraZeneca also holds a major stake in Moderna Therapeutics, which announced earlier this week its experimental vaccine had produced antibodies in small group of healthy volunteers. -
 2021-10-22
2021-10-22HIST30060 The First Night Out!
This is an image I took on the first night out of the last Melbourne lockdown! I went to my favourite pasta place in Melbourne for dinner and this was their way of making sure everyone they served was fully vaccinated and checked in. Once checking in the server gave us this little laminated slip we gave to the waiter in return for the menu, this ensured no one had sat down without checking in. In Melbourne, it is mandatory for all hospitality venues to have their staff and customers fully vaccinated. -
2021-11-02
COVID-19 Pandemic
The text story would be about my feelings toward COVID-19. It is sad that I don't remember much of what life was like before the pandemic hit. The masks, the social distancing, the looks you get when you have a runny nose. There are so many theories in regards to COVID-19 and much like anything else, everyone has their own opinion. Some revolve their lives around it, others don't care about. Personally, I'm not scared of the pandemic, I'm not vaccinated, nor do I get worried when people around me test positive for it. It is important to me because it has significantly changed our lives for the worse. We miss out on extremely important events and experiences. In my opinion, much of this should be a choice. If you want to wear a mask, wear one. If you want to social distance, do it. If you want to get vaccinated, get vaccinated. -
 2021-03-05
2021-03-05First State Mass Vaccination Clinic
A screenshot of when my college announced that our basketball arena would house the first mass vaccination center in our state. I sent this to my family because I thought it was so cold my college got this opportunity. Most of my family got vaccinated here and it was so well run and easy! -
 2021-09-30
2021-09-30Pro-Vax, Pro-Union Anti-Fascist Poster - Jewish Melbourne
In the wake of the anti-lockdown riots that gripped Melbourne in September 2021, the Campaign Against Fascism movement disseminated the phrase Pro-Vax, Pro-Union, Anti-Fascist. Inspired by this, Link/לינק, a zine associated with the Jewish Labour Bund in Melbourne, posted a poster to their Instagram account, of their take on this messaging, including the shouting man from early twentieth-century Bund posters. The poster was also physically published in the zine's second edition in October 2021. HIST30060 -
 2021-08-19
2021-08-19Royal Exhibition Building: A COVID-19 Vaccination Centre
Pictured here is the Melbourne Royal Exhibition Building during its time as a COVID-19 Vaccination Centre. This building has had a plethora of purposes throughout its history. For me, it was once my university exam hall, and now in August 2021 it was the site of my first Pfizer COVID-19 vaccination. In this, one is given a glimpse into the transformations that the COVID-19 pandemic has brought to life in Melbourne. The city itself still exists, almost entirely unchanged, but the way we live our lives within it has been radically re-shaped by this pandemic. HIST30060 -
 2021-10-31
2021-10-31Health officials push for booster shots as 50% of last week’s COVID-19 deaths in Illinois were breakthrough cases
Health officials are pushing residents to get booster shots as state data shows about 50% of the recorded COVID deaths in Illinois last week were cases where people were fully vaccinated. The Illinois Department of Public Health (IDPH) reported there were 179 COVID-19 deaths from October 20 to October 27. From the same reporting period of October 20-27, there were 91 breakthrough cases, IDPH data shows. A breakthrough case is when a person tests positive for COVID-19 at least 14 days after being fully vaccinated and did not test positive in the previous 45 days, according to IDPH. 87% of all breakthrough COVID-19 deaths in Illinois have been in the 65+ age group. Illinois Department of Public Health spokeswoman Melaney Arnold told Lake and McHenry County Scanner that COVID-19 vaccines “continue to be highly effective at preventing death due to COVID-19.” [Suggested Article] Illinois attorney general, 51 other attorneys general call on Congress to protect children on Facebook, Instagram “Of the more than 7 million people in Illinois who are fully vaccinated, breakthrough deaths have occurred in 0.01% of the population,” Arnold said. Arnold noted that not all breakthrough deaths reported from October 20 to October 27 actually occurred in that span. “It takes time (days to weeks) to match death records with vaccination records; therefore; there can be a lag in when the death occurred and when it is reported as a breakthrough death,” she said. Still, the reporting week from October 20 to October 27 had the highest percent of breakthrough deaths compared to non-breakthrough deaths since the state began publishing the data back in April. Currently, Illinois residents 65 or older, anyone 18+ with underlying medical conditions or who work or live in high-risk settings, such as educators and first responders, are eligible to receive a booster shot of the Pfizer and Moderna vaccines. Those who received the Johnson and Johnson vaccine are able to get any COVID-19 vaccine as their booster shot, the CDC says. “We know that advanced age is a significant factor in COVID-19 breakthrough hospitalizations and deaths, but a booster dose can help provide continued protection,” IDPH Director Dr. Ngozi Ezike said earlier this month. “While COVID-19 vaccines continue to be effective in reducing the risk of severe disease, hospitalization, and death, scientists and medical experts continue to watch for signs of waning immunity, how well the vaccines protect against variants, and how that data differs across age groups and risk factors,” Ezike said. -
 2021-09-07
2021-09-07The Possibility of COVID-19 after Vaccination: Breakthrough Infections
COVID-19 vaccines are effective at preventing infection, serious illness, and death. Most people who get COVID-19 are unvaccinated. However, since vaccines are not 100% effective at preventing infection, some people who are fully vaccinated will still get COVID-19. An infection of a fully vaccinated person is referred to as a “vaccine breakthrough infection.”
